Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-01-09 , DOI: 10.1016/j.tetlet.2020.152797 Shijie Wang , Huiqin Wang , Nana Tian , Hong Yan
|
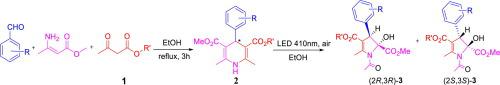
Strategies by introducing chiral auxiliaries into the photoreactive substrate 1,4-dihydropyridines, an interesting diastereoselectivity of 2,3-dihydropyrroles in the process of photochemical ring contraction was observed. The diastereoselectivity of (2R,3R) and (2S,3S)-2,3-dihydropyrroles was related to the phenyl group and the chirality of C-4 in 1,4-dihydropyridines and similar to that of 1,4-dihydropyridines. The yields and diastereomeric excess of all obtained products supporting the experimental data were compared and discussed in theoretical calculations. A concise theoretical study was used to explain the diastereoselectivity observed in the photochemical ring contraction of 1,4-dihydropyridines to 2,3-dihydropyrroles.
中文翻译:

1,4-二氢吡啶的光化学环收缩合成2,3-二氢吡咯的非对映选择性的见解
通过将手性助剂引入光反应性底物1,4-二氢吡啶中的策略,观察到在光化学环收缩过程中2,3-二氢吡咯具有有趣的非对映选择性。(2 R,3 R)和(2 S,3 S)-2,3-二氢吡咯的非对映选择性与1,4-二氢吡啶中的苯基和C-4的手性有关,与1, 4-二氢吡啶。比较了所有获得的支持实验数据的产物的产率和非对映异构体,并在理论计算中进行了讨论。简洁的理论研究用于解释在1,4-二氢吡啶对2,3-二氢吡咯的光化学环收缩中观察到的非对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号