Materials Today Energy ( IF 9.3 ) Pub Date : 2021-01-10 , DOI: 10.1016/j.mtener.2021.100646 Hao Chen , Shinan Cai , Yuanke Wu , Wei Wang , Maowen Xu , Shu-Juan Bao
|
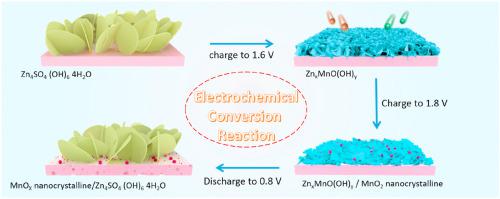
Rechargeable aqueous zinc-manganese oxide (Zn/MnO2) batteries using Mn2+ as the electrolyte additive have recently attracted remarkable attention owing to their largely improved cycling stability. Herein, we find that the Zn/MnO2 batteries with Mn2+ additive still exhibit rapid capacity fading when cycling between 0.8 and 1.6 V vs. Zn/Zn2+, its improved performance is only observed when charged to a higher slope region (>1.6 V), which suggests that the improved performance of Mn2+ added Zn/MnO2 is not caused by suppressing the dissolution of MnO2 cathode. Inspired by this discovery, successive electrochemcial conversion reactions are scrutinized and proved for evaluating the performance of the Zn/MnO2 batteries after using Mn2+ as the electrolyte additive. By adding a certain amount of Mn2+ into the electrolyte, the battery can improve the capacity and cycling abilitily through converted electrodepostion of Mn2+. Specifically, the zinc sulfate hydroxide hydrate (Zn4SO4·(OH)6·4H2O) large-flake can initiate the generation of zinc vernadite nanosheets (ZnxMnO(OH)y) during the charge process (around 1.5 V vs. Zn/Zn2+), and then the zinc vernadite nanosheets can reversibly re-back to Zn4SO4·(OH)6·4H2O during the discharge process. Importantly, part of zinc vernadite nanosheets irreversibly transform into tunnel-like MnO2 nanocrystalline material when charging higher than 1.6 V vs. Zn/Zn2+, which can improve the specific capacity of Zn/MnO2 batteries in subsequent cycles and then make the Zn/MnO2 batteries exhibit excellent cycles stability. Finally, through a special zinc/carbon nanotube (Zn/CNT) battery, this successive electrochemcial conversion reactions are further verified.
中文翻译:

连续进行电化学转化反应,以了解含Mn 2+添加剂的Zn / MnO 2水性电池的性能
使用Mn 2+作为电解质添加剂的可再充电的含水锌锰氧化物(Zn / MnO 2)电池由于其循环稳定性的大幅提高,近来引起了人们的极大关注。在这里,我们发现具有Mn 2+添加剂的Zn / MnO 2电池在0.8至1.6 V的电压与Zn / Zn 2+之间循环时仍显示出快速的容量衰减,其性能只有在充电到较高的斜率区域时才能观察到( > 1.6 V),这表明添加Mn 2+的Zn / MnO 2的性能改善不是由于抑制MnO 2的溶解而引起的阴极。受这一发现的启发,对连续的电化学转化反应进行了仔细的考察,并证明了在使用Mn 2+作为电解质添加剂后可用于评估Zn / MnO 2电池的性能。通过将一定量的Mn 2+添加到电解液中,电池可以通过转换后的Mn 2+电极沉积来提高容量和稳定循环。具体地说,在充电过程中(约1.5 V),氢氧化锌水合物(Zn 4 SO 4 ·(OH)6 ·4H 2 O)大片状可以引发锌白云母纳米片(Zn x MnO(OH)y)的生成。与锌/锌2+),然后将锌水羟锰矿纳米片能够可逆地重新返回到锌4 SO 4 ·(OH)6 ·4H 2 ö在放电过程。重要的是,当锌与锌/ Zn 2+的充电电压高于1.6 V时,部分锌白云母纳米片不可逆地转变为隧道状MnO 2纳米晶体材料,这可以提高Zn / MnO 2电池在随后的循环中的比容量,然后使Zn / MnO 2电池具有出色的循环稳定性。最后,通过特殊的锌/碳纳米管(Zn / CNT)电池,进一步验证了这种连续的电化学转化反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号