Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-01-09 , DOI: 10.1016/j.bmc.2021.116013 Aiko Yamaguchi 1 , Yasuaki Anami 1 , Summer Y Y Ha 1 , Travis J Roeder 1 , Wei Xiong 1 , Jangsoon Lee 2 , Naoto T Ueno 2 , Ningyan Zhang 1 , Zhiqiang An 1 , Kyoji Tsuchikama 1
|
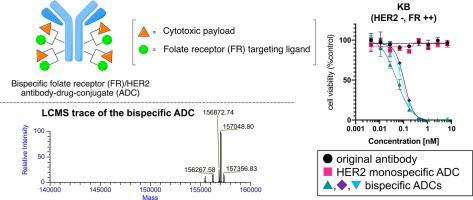
Antibody-drug conjugates (ADCs) hold great therapeutic promise for cancer indications; however, treating tumors with intratumor heterogeneity remains challenging. We hypothesized that ADCs that can simultaneously target two different cancer antigens could address this issue. Here, we report controlled production and evaluation of bispecific ADCs chemically functionalized with tumor-targeting small molecules. Enzyme-mediated conjugation of bi-functional branched linkers and following sequential orthogonal click reactions with payload and tumor targeting modules (folic acid or RGD peptide) afforded homogeneous bispecific ADCs with defined ligand/drug-to-antibody ratios ranging from 4 + 4 to 16 + 4 (ligand/payload). Most bispecific ADCs were stable under physiological conditions for 14 days. Functionalization with the cancer-specific ligands did not impair cathepsin B-mediated payload release from ADCs. Bispecific ADCs targeting the folate receptor (FR)/human epidermal growth factor receptor 2 (HER2) demonstrated specific binding and high cell killing potency only in cells expressing either antigen (FR or HER2). Integrin/HER2 bispecific ADCs equipped with RGD peptides also showed target-specific binding and cytotoxicity in integrin- or HER2-positive cells. These findings suggest that our small-molecule based bispecific ADCs have the potential to effectively treat tumors with heterogeneous antigen expression.
中文翻译:

基于小分子的双特异性抗体-药物偶联物的化学生成以扩大目标范围
抗体药物偶联物 (ADC) 对癌症适应症具有巨大的治疗前景;然而,治疗具有肿瘤内异质性的肿瘤仍然具有挑战性。我们假设可以同时靶向两种不同癌症抗原的 ADC 可以解决这个问题。在这里,我们报告了用靶向肿瘤的小分子化学功能化的双特异性 ADC 的受控生产和评估。双功能分支接头的酶介导缀合以及随后与有效载荷和肿瘤靶向模块(叶酸或 RGD 肽)的连续正交点击反应提供了均质的双特异性 ADC,其配体/药物与抗体的比例定义为 4 + 4 至 16 + 4(配体/有效载荷)。大多数双特异性 ADC 在生理条件下稳定 14 天。使用癌症特异性配体进行功能化不会损害 ADC 中组织蛋白酶 B 介导的有效载荷释放。靶向叶酸受体 (FR)/人表皮生长因子受体 2 (HER2) 的双特异性 ADC 仅在表达任一抗原(FR 或 HER2)的细胞中表现出特异性结合和高细胞杀伤效力。配备 RGD 肽的整合素 / HER2 双特异性 ADC 在整合素或 HER2 阳性细胞中也显示出靶标特异性结合和细胞毒性。这些发现表明,我们基于小分子的双特异性 ADC 具有有效治疗具有异质抗原表达的肿瘤的潜力。靶向叶酸受体 (FR)/人表皮生长因子受体 2 (HER2) 的双特异性 ADC 仅在表达任一抗原(FR 或 HER2)的细胞中表现出特异性结合和高细胞杀伤效力。配备 RGD 肽的整合素 / HER2 双特异性 ADC 在整合素或 HER2 阳性细胞中也显示出靶标特异性结合和细胞毒性。这些发现表明,我们基于小分子的双特异性 ADC 具有有效治疗具有异质抗原表达的肿瘤的潜力。靶向叶酸受体 (FR)/人表皮生长因子受体 2 (HER2) 的双特异性 ADC 仅在表达任一抗原(FR 或 HER2)的细胞中表现出特异性结合和高细胞杀伤效力。配备 RGD 肽的整合素 / HER2 双特异性 ADC 在整合素或 HER2 阳性细胞中也显示出靶标特异性结合和细胞毒性。这些发现表明,我们基于小分子的双特异性 ADC 具有有效治疗具有异质抗原表达的肿瘤的潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号