当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Approaches for Assignment of the Relative Configuration in 1-Oxygenated 1,2-Diarylpropan-3-ols by 1H NMR Spectroscopy
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-01-08 , DOI: 10.1021/acs.jnatprod.0c00828 Le Zhou 1 , Rui Guo 1 , Han Zhang 1 , Li-Wei Lu 1 , Ye-Qing Du 1 , Qing-Bo Liu 1 , Xiao-Xiao Huang 1 , Shao-Jiang Song 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-01-08 , DOI: 10.1021/acs.jnatprod.0c00828 Le Zhou 1 , Rui Guo 1 , Han Zhang 1 , Li-Wei Lu 1 , Ye-Qing Du 1 , Qing-Bo Liu 1 , Xiao-Xiao Huang 1 , Shao-Jiang Song 1
Affiliation
|
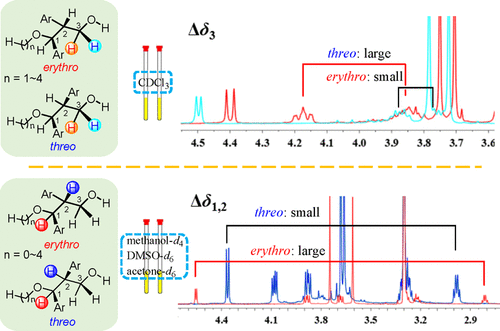
The structural elucidation of chiral molecules with more than one stereocenter is usually a tricky problem. In this paper, efficient 1H NMR spectroscopic approaches for assigning the erythro and threo configurations of 1-oxygenated 1,2-diarylpropan-3-ols were developed. By analysis of the chemical shift differences of diastereotopic methylene H2-3 (Δδ3) in CDCl3 or the chemical shift differences of H-1 and H-2 (Δδ1,2) in methanol-d4, deuterated dimethyl sulfoxide, and acetone-d6, the configurations of 1-oxygenated 1,2-diarylpropan-3-ols can be rapidly and conveniently determined.
中文翻译:

通过 1H NMR 光谱快速确定 1-氧化 1,2-二芳基丙-3-醇中的相对构型
具有多个立体中心的手性分子的结构解析通常是一个棘手的问题。在本文中,开发了用于指定1-氧化 1,2-二芳基丙-3-醇的赤型和苏型构型的有效1 H NMR 光谱方法。通过分析非对映异构亚甲基H 2 -3 (Δδ 3 )在CDCl 3 中的化学位移差异或H-1和H-2 (Δδ 1,2 )在甲醇-d 4、氘代二甲亚砜中的化学位移差异,和丙酮-d 6,可以快速方便地确定1-氧化的1,2-二芳基丙-3-醇的构型。
更新日期:2021-01-22
中文翻译:

通过 1H NMR 光谱快速确定 1-氧化 1,2-二芳基丙-3-醇中的相对构型
具有多个立体中心的手性分子的结构解析通常是一个棘手的问题。在本文中,开发了用于指定1-氧化 1,2-二芳基丙-3-醇的赤型和苏型构型的有效1 H NMR 光谱方法。通过分析非对映异构亚甲基H 2 -3 (Δδ 3 )在CDCl 3 中的化学位移差异或H-1和H-2 (Δδ 1,2 )在甲醇-d 4、氘代二甲亚砜中的化学位移差异,和丙酮-d 6,可以快速方便地确定1-氧化的1,2-二芳基丙-3-醇的构型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号