当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Navigating the Pauson–Khand Reaction in Total Syntheses of Complex Natural Products
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-01-07 , DOI: 10.1021/acs.accounts.0c00709 Zhen Yang 1, 2, 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-01-07 , DOI: 10.1021/acs.accounts.0c00709 Zhen Yang 1, 2, 3
Affiliation
|
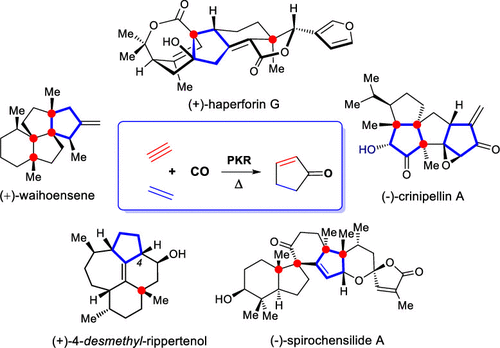
“Total synthesis endeavors provide wonderful opportunities to discover and invent new synthetic reactions as a means to advance organic synthesis in general. Such discoveries and inventions can occur when the practitioner faces intransigent problems that cannot be solved by known methods and/or when method improvements are desired in terms of elegance, efficiency, cost-effectiveness, practicality, or environmental friendliness” (K. C. Nicolaou et al. from their review in CCS Chem. 2019, 1, 3–37). To date tens of thousands of bioactive compounds have been isolated from plants, microbes, marine invertebrates, and other sources. These chemical structures have been studied by chemists who scanned the breadth of natural diversity toward drug discovery efforts. Drug-likeness of natural products often possesses common features including molecular complexity, protein-binding ability, structural rigidity, and three-dimensionality. Considering certain biologically important natural products are scarce from natural supply, total synthesis may provide an alternative solution to generating these compounds and their derivatives for the purpose of probing their biological functions. Natural products bearing quaternary carbon stereocenters represent a group of biologically important natural entities that are lead compounds in the development of pharmacological agents and biological probes. However, the stereocontrolled introduction of quaternary carbons, with vicinal patterns that substantially expand the complexity of molecular architectures and chemical space in particular, presents distinct challenges because of the high steric repulsion between substituents. Though remarkable advance has been seen for quaternary carbon stereocenter generation, the process remains a daunting challenge given that the formation of highly congested stereocenters increases the difficulty in achieving orbital overlap.
中文翻译:

在复杂天然产物的总合成中浏览Pauson-Khand反应
全面的合成努力为发现和发明新的合成反应提供了绝好的机会,以此作为总体上推进有机合成的手段。当从业人员面临无法通过已知方法解决的棘手问题和/或需要在美观,效率,成本效益,实用性或环境友好性方面进行方法改进时,就会发生这样的发现和发明”(KC Nicolaou等人。从他们的审核CCS化学。 2019,1,3–37)。迄今为止,已经从植物,微生物,海洋无脊椎动物和其他来源分离出数以万计的生物活性化合物。化学家已经研究了这些化学结构,他们扫描了自然多样性的广度以进行药物开发。天然产物的类药物通常具有共同的特征,包括分子复杂性,蛋白质结合能力,结构刚性和三维性。考虑到某些生物学上重要的天然产物缺乏天然供应,因此为了探测其生物学功能,全合成可为生成这些化合物及其衍生物提供另一种解决方案。带有季碳立体中心的天然产物代表一组生物学上重要的天然实体,它们是药理学试剂和生物探针开发中的先导化合物。然而,由于取代基之间的高空间排斥性,以立体方式引入的季碳具有明显扩展了分子结构尤其是化学空间的复杂性的邻近模式,提出了独特的挑战。尽管四元碳立体中心的产生已经取得了显着进展,但鉴于高度拥挤的立体中心的形成增加了实现轨道重叠的难度,这一过程仍然是艰巨的挑战。具有邻位模式的分子结构极大地扩展了分子结构的复杂性,尤其是化学空间,由于取代基之间的空间排斥力高,因此面临着独特的挑战。尽管四元碳立体中心的产生已经取得了显着进展,但鉴于高度拥挤的立体中心的形成增加了实现轨道重叠的难度,这一过程仍然是艰巨的挑战。具有邻位模式的分子结构极大地扩展了分子结构的复杂性,尤其是化学空间,由于取代基之间的空间排斥力高,因此面临着独特的挑战。尽管四元碳立体中心的产生已经取得了显着进展,但鉴于高度拥挤的立体中心的形成增加了实现轨道重叠的难度,这一过程仍然是艰巨的挑战。
更新日期:2021-02-02
中文翻译:

在复杂天然产物的总合成中浏览Pauson-Khand反应
全面的合成努力为发现和发明新的合成反应提供了绝好的机会,以此作为总体上推进有机合成的手段。当从业人员面临无法通过已知方法解决的棘手问题和/或需要在美观,效率,成本效益,实用性或环境友好性方面进行方法改进时,就会发生这样的发现和发明”(KC Nicolaou等人。从他们的审核CCS化学。 2019,1,3–37)。迄今为止,已经从植物,微生物,海洋无脊椎动物和其他来源分离出数以万计的生物活性化合物。化学家已经研究了这些化学结构,他们扫描了自然多样性的广度以进行药物开发。天然产物的类药物通常具有共同的特征,包括分子复杂性,蛋白质结合能力,结构刚性和三维性。考虑到某些生物学上重要的天然产物缺乏天然供应,因此为了探测其生物学功能,全合成可为生成这些化合物及其衍生物提供另一种解决方案。带有季碳立体中心的天然产物代表一组生物学上重要的天然实体,它们是药理学试剂和生物探针开发中的先导化合物。然而,由于取代基之间的高空间排斥性,以立体方式引入的季碳具有明显扩展了分子结构尤其是化学空间的复杂性的邻近模式,提出了独特的挑战。尽管四元碳立体中心的产生已经取得了显着进展,但鉴于高度拥挤的立体中心的形成增加了实现轨道重叠的难度,这一过程仍然是艰巨的挑战。具有邻位模式的分子结构极大地扩展了分子结构的复杂性,尤其是化学空间,由于取代基之间的空间排斥力高,因此面临着独特的挑战。尽管四元碳立体中心的产生已经取得了显着进展,但鉴于高度拥挤的立体中心的形成增加了实现轨道重叠的难度,这一过程仍然是艰巨的挑战。具有邻位模式的分子结构极大地扩展了分子结构的复杂性,尤其是化学空间,由于取代基之间的空间排斥力高,因此面临着独特的挑战。尽管四元碳立体中心的产生已经取得了显着进展,但鉴于高度拥挤的立体中心的形成增加了实现轨道重叠的难度,这一过程仍然是艰巨的挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号