Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-01-08 , DOI: 10.1016/j.bmc.2021.116011 Aiping Bai 1 , Jacek Bielawski 1 , Alicja Bielawska 1 , Yusuf A Hannun 2
|
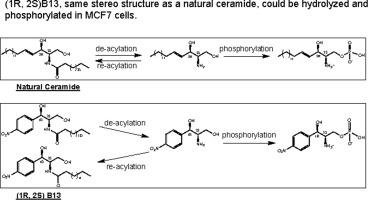
B13 is an acid ceramidase (ACDase) inhibitor. The two chiral centers of this aromatic amido alcohol lead to four stereoisomers, yet we have little knowledge about its erythro- enantiomers, (1R, 2S) and (1S, 2R). In this paper, for the first time, the synthesis of two erythro- enantiomers is described, and the compounds are evaluated along with two threo- enantiomers, (1R, 2R) and (1S, 2S). The key metabolites and sphingolipid (SL) profile of the full set of B13 stereoisomers in MCF7 breast carcinoma cells are presented. The results demonstrated that the erythro- enantiomers were more effective than the threo- enantiomers on growth inhibition in MCF7 cells, although there were no statistically significant differences within the threo- and erythro- series. Measurement of intracellular levels of the compounds indicated that the erythro- seemed a little more cell permeable than the threo- enantiomers; also, the (1R, 2S) isomer with the same stereo structure as natural ceramide (Cer) could be hydrolyzed and phosphorylated in MCF7 cells. Furthermore, we also observed the formation of C16 homologs from the full set of B13 isomers within the cells, indicating the occurrence of de-acylation and re-acylation of the amino group of the aromatic alcohol. Moreover, the decrease in the Cer/Sph ratio suggests that the growth inhibition from (1R, 2S) isomer is not because of the inhibition of ceramidases. Taken together, (1R, 2S) could be developed as a substitute of natural Cer.
中文翻译:

MCF7乳腺癌细胞中赤型B13对映体的合成和全套B13异构体的立体特异性作用:细胞代谢和对鞘脂的影响
B13 是一种酸性神经酰胺酶 (ACDase) 抑制剂。这种芳香酰胺醇的两个手性中心导致四种立体异构体,但我们对其赤式对映异构体(1R,2S)和(1S,2R)知之甚少。本文首次描述了两种赤型对映体的合成,并与两种赤型对映体 (1R, 2R) 和 (1S, 2S)一起评估了这些化合物。介绍了 MCF7 乳腺癌细胞中全套 B13 立体异构体的关键代谢物和鞘脂 (SL) 谱。结果表明,赤型对映体比苏型更有效。对映异构体对 MCF7 细胞的生长抑制作用,尽管在threo-和erythro-系列中没有统计学上的显着差异。化合物的细胞内水平的测量表明,红细胞似乎比苏细胞渗透性更强。对映体;此外,具有与天然神经酰胺(Cer)相同立体结构的(1R,2S)异构体可以在MCF7细胞中水解和磷酸化。此外,我们还观察到细胞内全套 B13 异构体形成 C16 同系物,表明芳香醇的氨基发生了去酰化和再酰化。此外,Cer/Sph 比率的降低表明(1R,2S)异构体的生长抑制不是因为神经酰胺酶的抑制。总之,(1R,2S)可以开发为天然Cer的替代品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号