当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembly of Silk-like Protein into Nanoscale Bicontinuous Networks under Phase-Separation Conditions
Biomacromolecules ( IF 5.5 ) Pub Date : 2021-01-06 , DOI: 10.1021/acs.biomac.0c01506 Piotr Batys 1, 2 , Dmitrii Fedorov 2 , Pezhman Mohammadi 3 , Laura Lemetti 2 , Markus B Linder 2 , Maria Sammalkorpi 2, 4
Biomacromolecules ( IF 5.5 ) Pub Date : 2021-01-06 , DOI: 10.1021/acs.biomac.0c01506 Piotr Batys 1, 2 , Dmitrii Fedorov 2 , Pezhman Mohammadi 3 , Laura Lemetti 2 , Markus B Linder 2 , Maria Sammalkorpi 2, 4
Affiliation
|
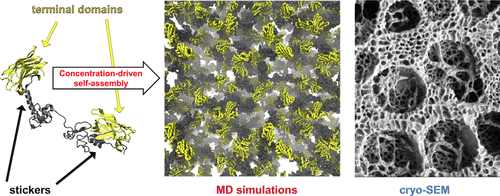
Liquid–liquid phase separation of biomacromolecules is crucial in various inter- and extracellular biological functions. This includes formation of condensates to control, e.g., biochemical reactions and structural assembly. The same phenomenon is also found to be critically important in protein-based high-performance biological materials. Here, we use a well-characterized model triblock protein system to demonstrate the molecular level formation mechanism and structure of its condensate. Large-scale molecular modeling supported by analytical ultracentrifuge characterization combined with our earlier high magnification precision cryo-SEM microscopy imaging leads to deducing that the condensate has a bicontinuous network structure. The bicontinuous network rises from the proteins having a combination of sites with stronger mutual attraction and multiple weakly attractive regions connected by flexible, multiconfigurational linker regions. These attractive sites and regions behave as stickers of varying adhesion strength. For the examined model triblock protein construct, the β-sheet-rich end units are the stronger stickers, while additional weaker stickers, contributing to the condensation affinity, rise from spring-like connections in the flexible middle region of the protein. The combination of stronger and weaker sticker-like connections and the flexible regions between the stickers result in a versatile, liquid-like, self-healing structure. This structure also explains the high flexibility, easy deformability, and diffusion of the proteins, decreasing only 10–100 times in the bicontinuous network formed in the condensate phase in comparison to dilute protein solution. The here demonstrated structure and condensation mechanism of a model triblock protein construct via a combination of the stronger binding regions and the weaker, flexible sacrificial-bond-like network as well as its generalizability via polymer sticker models provide means to not only understand intracellular organization, regulation, and cellular function but also to identify direct control factors for and to enable engineering improved protein and polymer constructs to enhance control of advanced fiber materials, smart liquid biointerfaces, or self-healing matrices for pharmaceutics or bioengineering materials.
中文翻译:

在相分离条件下丝状蛋白自组装成纳米级双连续网络
生物大分子的液-液相分离在各种细胞间和细胞外生物学功能中至关重要。这包括形成冷凝物以控制例如生化反应和结构组装。在基于蛋白质的高性能生物材料中,同样的现象也被发现至关重要。在这里,我们使用特征充分的三嵌段蛋白系统模型来证明其缩合物的分子水平形成机理和结构。分析超速离心表征与我们较早的高倍率精密冰冻-SEM显微镜成像相结合支持的大规模分子建模可以推断出冷凝液具有双连续网络结构。双连续网络是由蛋白质组成的,蛋白质具有相互吸引的位点和通过柔性多构型接头区域连接的多个弱吸引区。这些吸引人的部位和区域表现为不同粘合强度的标签。对于所研究的模型三嵌段蛋白质构建体,富含β-折叠的末端单元是较强的粘着剂,而其他较弱的粘着剂则是从蛋白质的柔性中间区域中的弹簧状连接处产生的,从而有助于缩合亲和力。较强和较弱的类似粘贴物的连接以及粘贴物之间的柔性区域的结合,形成了一种通用的,类似液体的自愈结构。这种结构还解释了蛋白质的高柔韧性,易变形性和扩散性,与稀蛋白溶液相比,在凝结相中形成的双连续网络中仅降低10-100倍。这里通过结合更强的结合区域和更弱的,灵活的牺牲键样网络,展示了模型三嵌段蛋白构建体的结构和缩合机理,以及通过聚合物标签模型的泛化性,不仅提供了理解细胞内组织的手段,调节和细胞功能,还可以识别直接的控制因素,并使工程改造的蛋白质和聚合物构建体能够增强对高级纤维材料,智能液体生物界面或药物或生物工程材料的自愈基质的控制。
更新日期:2021-02-08
中文翻译:

在相分离条件下丝状蛋白自组装成纳米级双连续网络
生物大分子的液-液相分离在各种细胞间和细胞外生物学功能中至关重要。这包括形成冷凝物以控制例如生化反应和结构组装。在基于蛋白质的高性能生物材料中,同样的现象也被发现至关重要。在这里,我们使用特征充分的三嵌段蛋白系统模型来证明其缩合物的分子水平形成机理和结构。分析超速离心表征与我们较早的高倍率精密冰冻-SEM显微镜成像相结合支持的大规模分子建模可以推断出冷凝液具有双连续网络结构。双连续网络是由蛋白质组成的,蛋白质具有相互吸引的位点和通过柔性多构型接头区域连接的多个弱吸引区。这些吸引人的部位和区域表现为不同粘合强度的标签。对于所研究的模型三嵌段蛋白质构建体,富含β-折叠的末端单元是较强的粘着剂,而其他较弱的粘着剂则是从蛋白质的柔性中间区域中的弹簧状连接处产生的,从而有助于缩合亲和力。较强和较弱的类似粘贴物的连接以及粘贴物之间的柔性区域的结合,形成了一种通用的,类似液体的自愈结构。这种结构还解释了蛋白质的高柔韧性,易变形性和扩散性,与稀蛋白溶液相比,在凝结相中形成的双连续网络中仅降低10-100倍。这里通过结合更强的结合区域和更弱的,灵活的牺牲键样网络,展示了模型三嵌段蛋白构建体的结构和缩合机理,以及通过聚合物标签模型的泛化性,不仅提供了理解细胞内组织的手段,调节和细胞功能,还可以识别直接的控制因素,并使工程改造的蛋白质和聚合物构建体能够增强对高级纤维材料,智能液体生物界面或药物或生物工程材料的自愈基质的控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号