当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convergent Assembly of Highly Oxygenated Natural Products Enabled by Intermolecular Radical Reactions
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-01-06 , DOI: 10.1021/acs.accounts.0c00792 Masanori Nagatomo 1 , Masayuki Inoue 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-01-06 , DOI: 10.1021/acs.accounts.0c00792 Masanori Nagatomo 1 , Masayuki Inoue 1
Affiliation
|
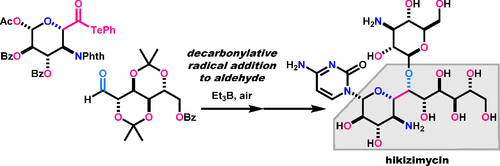
Natural products with a high ratio of sp3-hybridized atoms and oxygen-substituted stereogenic centers represent privileged structures for the development of pharmaceuticals and chemical probes. The multiple oxygen functionalities of these natural products endow their potent and selective biological activities, although they significantly heighten the challenge of their chemical assemblies. We focused on developing efficient strategies for the total syntheses of this biologically and chemically important class of molecules. A convergent strategy is more advantageous than a linear strategy for designing a shorter synthetic route because a convergent strategy enables direct coupling of functionalized fragments whereas a linear strategy involves stepwise construction of a molecule from its terminus. Radical reactions are preferred over polar reactions for the coupling of heavily functionalized and sp3-rich fragments, as they allow for C(sp3)–C(sp3) coupling without damaging diverse polar functional groups. With these considerations in mind, we designed radical-based convergent strategies for assembling highly oxygenated natural products. Here we summarize the concise total syntheses of asimicin (1, antibiotic activity), 1-hydroxytaxinine (2, cytotoxicity), polyoxins (3, antifungal activity), and hikizimycin (4, anthelmintic activity) as representative examples. Retrosynthetic disconnection at the central part of these molecules produces highly substituted α-alkoxy radicals as synthons. In the synthetic direction, the α-alkoxy radicals were generated from the corresponding α-alkoxyacyl tellurides in a unified fashion, and then utilized for four distinct coupling reactions. Formation of the Et radical from Et3B and O2 homolytically cleaves the C–Te bond of α-alkoxyacyl telluride, and the facile expulsion of carbon monoxide from the acyl radical leads to the α-alkoxy radical. Dimerization of the stabilized α-alkoxy radical resulted in the core structure of 1 with 10 contiguous stereocenters. The coupling adduct was derivatized to 1 through the attachment of two different carbon chains (17 steps as the longest linear sequence). Alternatively, intermolecular addition reactions of the α-alkoxy radicals to electron-deficient C═C, C═N, and C═O bonds, followed by Et3B-mediated radical termination, led to the core structures of 2, 3, and 4, respectively. Intermolecular coupling between the α-alkoxy radical and the cyclohexenone derivative and intramolecular pinacol coupling gave rise to the 6/8/6-fused ring system of 2, which was transformed to 2 (26 steps). The two amino acid moieties of 3 were prepared by combining the α-alkoxy radical and the oxime and were then condensed to complete the synthesis of 3 (11 steps). Furthermore, a combination of α-alkoxyacyl telluride and Et3B/O2 realized a novel addition reaction of α-alkoxy radicals to aldehydes. This method was incorporated in the construction of the core 4-amino-5-deoxyundecose with 10 contiguous stereocenters, which was fabricated with two appendage structures to deliver 4. The four total syntheses described here demonstrate the versatility and robustness of intermolecular radical reactions. These syntheses will also provide new insights for retrosynthetic analyses in the field of organic chemistry and streamline synthetic routes to various bioactive natural products with multiple oxygen functionalities.
中文翻译:

分子间自由基反应实现的高氧天然产物的会聚组装
天然产物的sp 3比例高杂原子和氧取代的立体异构中心代表了开发药物和化学探针的优先结构。这些天然产物的多种氧气功能赋予了它们强大的和选择性的生物活性,尽管它们大大增加了其化学组装的挑战。我们致力于为这种生物学和化学上重要的分子的总合成开发有效的策略。对于设计较短的合成路线,收敛策略比线性策略更具优势,因为收敛策略可实现功能化片段的直接偶联,而线性策略则涉及从其末端逐步构建分子。富含3的片段,因为它们允许C(sp 3)–C(sp 3)偶联而不会破坏各种极性官能团。考虑到这些考虑因素,我们设计了基于自由基的聚合策略来组装高度氧化的天然产物。在这里,我们总结了简明的总合成方法:阿司米星(1,具有抗生素活性),1-羟基紫杉碱(2,具有细胞毒性),多毒素(3,抗真菌活性)和喜霉素(4,例如驱虫药)。这些分子中心部分的逆合成断开产生高度取代的α-烷氧基自由基作为合成子。在合成方向上,以统一的方式从相应的α-烷氧基酰基碲化物生成α-烷氧基自由基,然后将其用于四个不同的偶联反应。由Et 3 B和O 2形成Et自由基会同质地裂解α-烷氧基酰基碲化物的C–Te键,并且容易地从酰基自由基中排出一氧化碳,从而形成α-烷氧基自由基。稳定的α-烷氧基的二聚化导致1的核心结构具有10个连续的立体中心。偶联加合物衍生为1通过两个不同碳链的连接(最长的线性序列为17个步骤)。可替换地,α-烷氧基的分子间加成反应的缺电子C = C,C = N,C = O和债券,随后的Et 3 B-介导的自由基终止,导致的芯结构2,3,和4。α-烷氧基和环己烯酮衍生物与分子内频哪醇偶联之间的分子间的耦合引起了的6/8/6-稠环体系2,将其转化为2(26步)。的两个氨基酸部分3通过结合α-烷氧基和肟制得化合物,然后缩合以完成3的合成(11个步骤)。此外,α-烷氧基酰基碲化物和Et 3 B / O 2的组合实现了α-烷氧基自由基与醛的新型加成反应。此方法被并入具有10个连续立体中心的核心4-氨基-5-脱氧十一糖的构建中,该构型由两个附肢结构制成,可递送4个。这里描述的四个总合成证明了分子间自由基反应的多功能性和鲁棒性。这些合成方法还将为有机化学领域的逆向合成分析提供新的见解,并简化合成路线以生产具有多种氧气功能的各种生物活性天然产物。
更新日期:2021-02-02
中文翻译:

分子间自由基反应实现的高氧天然产物的会聚组装
天然产物的sp 3比例高杂原子和氧取代的立体异构中心代表了开发药物和化学探针的优先结构。这些天然产物的多种氧气功能赋予了它们强大的和选择性的生物活性,尽管它们大大增加了其化学组装的挑战。我们致力于为这种生物学和化学上重要的分子的总合成开发有效的策略。对于设计较短的合成路线,收敛策略比线性策略更具优势,因为收敛策略可实现功能化片段的直接偶联,而线性策略则涉及从其末端逐步构建分子。富含3的片段,因为它们允许C(sp 3)–C(sp 3)偶联而不会破坏各种极性官能团。考虑到这些考虑因素,我们设计了基于自由基的聚合策略来组装高度氧化的天然产物。在这里,我们总结了简明的总合成方法:阿司米星(1,具有抗生素活性),1-羟基紫杉碱(2,具有细胞毒性),多毒素(3,抗真菌活性)和喜霉素(4,例如驱虫药)。这些分子中心部分的逆合成断开产生高度取代的α-烷氧基自由基作为合成子。在合成方向上,以统一的方式从相应的α-烷氧基酰基碲化物生成α-烷氧基自由基,然后将其用于四个不同的偶联反应。由Et 3 B和O 2形成Et自由基会同质地裂解α-烷氧基酰基碲化物的C–Te键,并且容易地从酰基自由基中排出一氧化碳,从而形成α-烷氧基自由基。稳定的α-烷氧基的二聚化导致1的核心结构具有10个连续的立体中心。偶联加合物衍生为1通过两个不同碳链的连接(最长的线性序列为17个步骤)。可替换地,α-烷氧基的分子间加成反应的缺电子C = C,C = N,C = O和债券,随后的Et 3 B-介导的自由基终止,导致的芯结构2,3,和4。α-烷氧基和环己烯酮衍生物与分子内频哪醇偶联之间的分子间的耦合引起了的6/8/6-稠环体系2,将其转化为2(26步)。的两个氨基酸部分3通过结合α-烷氧基和肟制得化合物,然后缩合以完成3的合成(11个步骤)。此外,α-烷氧基酰基碲化物和Et 3 B / O 2的组合实现了α-烷氧基自由基与醛的新型加成反应。此方法被并入具有10个连续立体中心的核心4-氨基-5-脱氧十一糖的构建中,该构型由两个附肢结构制成,可递送4个。这里描述的四个总合成证明了分子间自由基反应的多功能性和鲁棒性。这些合成方法还将为有机化学领域的逆向合成分析提供新的见解,并简化合成路线以生产具有多种氧气功能的各种生物活性天然产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号