当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Complexing Additives on the Reversible Deposition/Dissolution of Magnesium in an Ionic Liquid
ChemElectroChem ( IF 3.5 ) Pub Date : 2021-01-07 , DOI: 10.1002/celc.202001488 Isabella Weber 1 , Johannes Ingenmey 2 , Johannes Schnaidt 1 , Barbara Kirchner 2 , R. Juergen Behm 3
ChemElectroChem ( IF 3.5 ) Pub Date : 2021-01-07 , DOI: 10.1002/celc.202001488 Isabella Weber 1 , Johannes Ingenmey 2 , Johannes Schnaidt 1 , Barbara Kirchner 2 , R. Juergen Behm 3
Affiliation
|
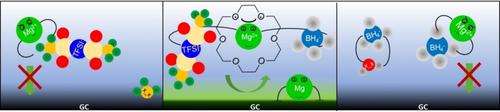
Aiming at a fundamental understanding of the synergistic effects of different additives on the electrochemical Mg deposition/dissolution in an ionic liquid, we have systematically investigated these processes in a combined electrochemical and theoretical study, using 1‐butyl‐1‐methylpyrrolidinium bis(trifluoromethylsulfonyl) imide (BMP‐TFSI) as the solvent and a cyclic ether (18‐crown‐6) and magnesium borohydride as additives. Both crown ether and BH4− improve Mg deposition, its reversibility, and cycling stability. The combined presence of both additives and their concentration relative to that of Mg2+ are decisive for more facile and reversible Mg deposition/dissolution. These results and those of quantum chemical calculations indicate that 18‐crown‐6 can partly displace TFSI− from its direct coordination to Mg2+. Furthermore, the interaction between Mg2+ and directly coordinated TFSI− is weakened by coordination with 18‐crown‐6, preventing its Mg+‐induced decomposition. Finally, Mg deposition is improved by the weaker overall coordination upon Mg2+ reduction to Mg+.
中文翻译:

络合添加剂对离子液体中镁的可逆沉积/溶解的影响
为了对不同添加剂对离子液体中电化学Mg沉积/溶解的协同作用有基本的了解,我们使用1-丁基-1-甲基吡咯烷鎓双(三氟甲基磺酰基)对电化学和理论研究进行了系统的研究酰亚胺(BMP-TFSI)作为溶剂,环醚(18-crown-6)和硼氢化镁作为添加剂。两个冠醚和BH 4 -提高镁沉积,其可逆性和循环稳定性。两种添加剂的组合存在及其相对于Mg 2+的浓度对于更容易和可逆地沉积/溶解镁至关重要。这些结果和量子化学计算的表明,18冠6可以部分取代TFSI -从它的直接协调与Mg 2+。此外,镁之间的相互作用2+协调TFSI和直接-通过配位与18-冠-6,防止其镁减弱+诱导的分解。最后,在将Mg 2+还原为Mg +时,通过较弱的整体配比可以改善Mg的沉积。
更新日期:2021-01-26
中文翻译:

络合添加剂对离子液体中镁的可逆沉积/溶解的影响
为了对不同添加剂对离子液体中电化学Mg沉积/溶解的协同作用有基本的了解,我们使用1-丁基-1-甲基吡咯烷鎓双(三氟甲基磺酰基)对电化学和理论研究进行了系统的研究酰亚胺(BMP-TFSI)作为溶剂,环醚(18-crown-6)和硼氢化镁作为添加剂。两个冠醚和BH 4 -提高镁沉积,其可逆性和循环稳定性。两种添加剂的组合存在及其相对于Mg 2+的浓度对于更容易和可逆地沉积/溶解镁至关重要。这些结果和量子化学计算的表明,18冠6可以部分取代TFSI -从它的直接协调与Mg 2+。此外,镁之间的相互作用2+协调TFSI和直接-通过配位与18-冠-6,防止其镁减弱+诱导的分解。最后,在将Mg 2+还原为Mg +时,通过较弱的整体配比可以改善Mg的沉积。











































 京公网安备 11010802027423号
京公网安备 11010802027423号