Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2021-01-07 , DOI: 10.1016/j.psep.2021.01.005 Gizele D. Silva , Eduardo O. Marson , Letícia L. Batista , Carlos Ueira-Vieira , Maria Clara V.M. Starling , Alam G. Trovó
|
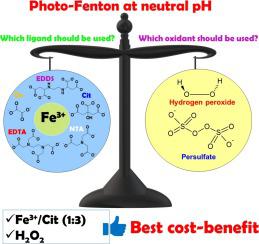
This is the first study to compare the combination of different iron complexes (Fe3+-oxalate (FeOx), Fe3+-citrate (FeCit), Fe3+-nitrilotriacetic acid (Fe3+-NTA), Fe3+-ethylenediaminetetraacetic acid (Fe3+-EDTA) and Fe3+-ethylenediamine-N,N´-disuccinic acid (Fe3+-EDDS) with distinct oxidants (H2O2 and S2O82―) on the degradation of naproxen (NAP) via photo-Fenton. Experiments were performed in distilled water and in sewage treatment plant effluent and different Fe/organic ligand molar ratios, oxidant concentrations and radiation sources (black light and sunlight) were tested for each complex. Photo-Fenton at neutral pH was efficient for naproxen degradation in the presence of all iron complexes. Fe/ligand molar ratio was strongly affected by the ligand type, best results were obtained in the presence of Fe/EDDS (1:1) and Fe/NTA (1:1), Fe/EDTA (1:2), Fe/Cit (1:3) and Fe/Ox (1:12). Although NAP removal in water was faster in the presence of H2O2 (max20 kJ m―2 required) when compared to S2O82― (max90 kJ m―2 required), better performance of S2O82― was observed in sewage treatment plant effluent. Antimicrobial activity was only observed in the presence of FeEDDS, yet it was eliminated after treatment in the presence of S2O82―. A critical comparison in terms of operational and electrical energy costs indicates that using FeCit complex with H2O2 is the most cost-effective alternative in both matrices.
中文翻译:

在过氧化氢或过硫酸盐作为氧化剂用于萘普生降解和去除抗菌活性的不同有机铁配合物存在下,对比光芬顿在中性pH下的性能
这是第一次研究不同铁络合物的Fe的组合(比较3+ -oxalate(的FeOx),铁3+柠檬酸(FeCit),铁3+次氮基三乙酸(铁3+ -NTA),铁3+ -乙二胺四乙酸(Fe 3+ -EDTA)和Fe 3+-乙二胺-N,N′-二琥珀酸(Fe 3+ -EDDS),具有不同的氧化剂(H 2 O 2和S 2 O 8 2―)通过光芬顿降解萘普生(NAP)。在蒸馏水和污水处理厂废水中进行了实验,并对每种配合物测试了不同的铁/有机配体摩尔比,氧化剂浓度和辐射源(黑光和阳光)。在所有铁络合物存在下,中性pH下的光芬顿对萘普生的降解有效。Fe /配体摩尔比受配体类型的影响很大,在Fe / EDDS(1:1)和Fe / NTA(1:1),Fe / EDTA(1:2),Fe /引用(1:3)和Fe / Ox(1:12)。虽然NAP去除水呈较快在H存在2 Ô 2(千焦耳MAX20米-2需要)相比至S时2 ö 8 2-(需要最大90 kJ m ―2),在污水处理厂废水中观察到了更好的S 2 O 8 2―性能。仅在FeEDDS存在下才观察到抗菌活性,但在S 2 O 8 2―存在下处理后却被消除。关于运行和电能成本的关键比较表明,在两种基质中,将FeCit配合物与H 2 O 2一起使用是最具成本效益的选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号