Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2021-01-07 , DOI: 10.1016/j.jphotochem.2021.113132 Mohamed Elhadi Benssassi , Lamia Mammeri , Tahar Sehili , Moisés Canle
|
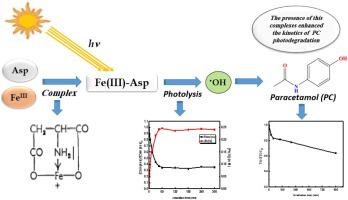
In this study, the photochemical properties of Fe(III)-Aspartate complex (Fe(III)-Asp) and its performance for the degradation of paracetamol (PC) in aqueous solution under UVA irradiation has been investigated. First, the Fe(III)-Aspartate complex was characterized using UV–vis absorption; the results confirmed that the complex was stable (Log β = 13.81), with molar ratio Fe(III) : Asp = 1:1. Upon irradiation, the Fe(III)-Asp complex undergoes photolysis leading to the formation of reactive oxygen species (ROS) such as HO . Moreover, this photochemical transformation is dependent on the irradiation wavelength and pH. The direct photolysis of paracetamol after 300 min under UVA irradiation showed a negligible effect, while 80 %, 59 %, and 15 % of paracetamol was eliminated in the presence of Fe(III)-Asp complex at pH = 2.05, 3.10, and 4.05; respectively. The degradation was investigated using various parameters such as initial pH, complex concentration, Fe(III):Asp molar ratio, and paracetamol concentration. The use of tert-butyl alcohol as a scavenger confirmed the participation of HO
. Moreover, this photochemical transformation is dependent on the irradiation wavelength and pH. The direct photolysis of paracetamol after 300 min under UVA irradiation showed a negligible effect, while 80 %, 59 %, and 15 % of paracetamol was eliminated in the presence of Fe(III)-Asp complex at pH = 2.05, 3.10, and 4.05; respectively. The degradation was investigated using various parameters such as initial pH, complex concentration, Fe(III):Asp molar ratio, and paracetamol concentration. The use of tert-butyl alcohol as a scavenger confirmed the participation of HO in paracetamol elimination. Addition of Zn (II) and Mn (II) to the system paracetamol-Fe(III)-Asp/UVA showed no significant effect on the degradation, however, Cu (II) had a slightly inhibiting effect. This study highlights the important role that organic iron complexes can play on the fate of pharmaceutical pollutants in aquatic environments.
in paracetamol elimination. Addition of Zn (II) and Mn (II) to the system paracetamol-Fe(III)-Asp/UVA showed no significant effect on the degradation, however, Cu (II) had a slightly inhibiting effect. This study highlights the important role that organic iron complexes can play on the fate of pharmaceutical pollutants in aquatic environments.
中文翻译:

光化学过程的初步证据,包括天冬氨酸铁络合物及其在水溶液中对乙酰氨基酚的消除
在这项研究中,研究了Fe(III)-天冬氨酸配合物(Fe(III)-Asp)的光化学性质及其在UVA照射下降解水溶液中对乙酰氨基酚(PC)的性能。首先,利用紫外可见吸收对Fe(III)-天冬氨酸配合物进行表征。结果证实该配合物是稳定的(Logβ= 13.81),摩尔比为Fe(III):Asp = 1:1。辐照后,Fe(III)-Asp复合物发生光解,导致形成活性氧(ROS),例如HO 。而且,这种光化学转化取决于照射波长和pH。对乙酰氨基酚在UVA辐射下300分钟后的直接光解作用可忽略不计,而在pH = 2.05、3.10和4.05的Fe(III)-Asp络合物存在下,扑热息痛的80%,59%和15%的扑热息痛被消除了; 分别。使用各种参数(例如初始pH,复合物浓度,Fe(III):Asp摩尔比和对乙酰氨基酚浓度)研究了降解情况。使用叔丁醇作为清除剂证实了HO的参与
。而且,这种光化学转化取决于照射波长和pH。对乙酰氨基酚在UVA辐射下300分钟后的直接光解作用可忽略不计,而在pH = 2.05、3.10和4.05的Fe(III)-Asp络合物存在下,扑热息痛的80%,59%和15%的扑热息痛被消除了; 分别。使用各种参数(例如初始pH,复合物浓度,Fe(III):Asp摩尔比和对乙酰氨基酚浓度)研究了降解情况。使用叔丁醇作为清除剂证实了HO的参与 在扑热息痛消除中。在对乙酰氨基酚-Fe(III)-Asp / UVA体系中添加Zn(II)和Mn(II)对降解没有明显影响,而Cu(II)则具有轻微的抑制作用。这项研究强调了有机铁配合物在水生环境中对制药污染物的命运起着重要作用。
在扑热息痛消除中。在对乙酰氨基酚-Fe(III)-Asp / UVA体系中添加Zn(II)和Mn(II)对降解没有明显影响,而Cu(II)则具有轻微的抑制作用。这项研究强调了有机铁配合物在水生环境中对制药污染物的命运起着重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号