Journal of Catalysis ( IF 6.5 ) Pub Date : 2021-01-07 , DOI: 10.1016/j.jcat.2020.12.019 Yuanyuan Yue , Jing Fu , Chuanming Wang , Pei Yuan , Xiaojun Bao , Zailai Xie , Jean-Marie Basset , Haibo Zhu
|
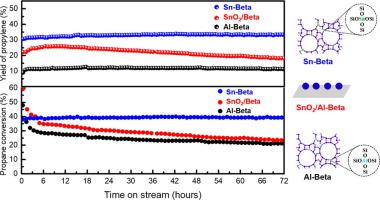
The gap between supply and demand of propylene has become more and more evident, because of a large consumption of the downstream products derived from propylene. Propane dehydrogenation (PDH) constitutes an important alternative for the production of propylene, and thus considerable attention has been paid to the development of eco-friendly and cost-efficient catalysts for this process. Herein, we discover that the Sn-Beta zeolite with Lewis acid sites can activate the C-H bond, and exhibits high catalytic performance in the PDH. XRD, STEM, and XPS characterizations confirm that Sn species are incorporated into the zeolite framework, and H2-TPR suggests that there is a strong interaction between Sn species and zeolite framework. It is found that the Lewis acid is the active site for dehydrogenation reaction, and the Brønsted acid is responsible for cracking reaction. The dehydrogenation rate/cracking rate is positively proportional to the L/B ratio, and a high L/B ratio is beneficial for the propane dehydrogenation reaction. The Na-Sn-Beta-30 catalyst possessing the highest amount of Lewis acid but the lowest Brønsted/Lewis ratio, exhibits the best performance in the PDH, which delivers propane conversion of 40% and propylene selectivity of 92%. Most importantly, these Sn-Beta zeolites are extremely stable without any detectable deactivation under the harsh reaction condition for 72 h. Density functional theory calculations reveal that both Sn and adjacent O atom or OH group cooperatively act as the active sites. The PDH occurs through the direct reaction mechanism in which hydrogen molecule is produced by the direct coupling of H atom of primary C3H7 motif with the Brønsted proton in closed sites or the proton of water in open sites. It seems that open sites are more reactive than the closed ones, and the intrinsic enthalpy barriers are calculated to be 242 ~ 301 kJ/mol depending on the hydroxylation extents. These efficient Sn-Beta zeolites could provide a new possibility for the development of a new generation of PDH catalysts with a high stability for the production of propylene.
中文翻译:

Sn-β沸石中单路易斯酸位催化丙烷脱氢
由于来自丙烯的下游产物的大量消耗,丙烯的供需之间的差距变得越来越明显。丙烷脱氢(PDH)构成了丙烯生产的重要替代方法,因此,人们已经相当重视开发用于该方法的环保且经济高效的催化剂。在本文中,我们发现具有路易斯酸位的Sn-Beta沸石可以激活CH键,并在PDH中表现出高催化性能。XRD,STEM和XPS表征证实,Sn物种被掺入沸石骨架中,而H 2-TPR表明,Sn物种与沸石骨架之间存在强相互作用。发现路易斯酸是脱氢反应的活性位点,而布朗斯台德酸负责裂化反应。脱氢率/裂化率与L / B比成正比,高L / B比对丙烷脱氢反应有利。Na-Sn-Beta-30催化剂具有最高的路易斯酸含量,但Brønsted/ Lewis比例最低,在PDH中表现出最好的性能,丙烷转化率为40%,丙烯选择性为92%。最重要的是,这些Sn-Beta沸石非常稳定,在苛刻的反应条件下持续72小时没有发现任何失活。 充当活动站点。PDH通过直接反应机理产生,在该机理中,氢原子是通过伯C 3 H 7基序的H原子与布朗斯台德质子在封闭位点或水质子在开放位点的直接偶联而产生的。似乎开放的位点比封闭的位点更具反应性,根据羟基化程度,固有的焓垒被计算为242〜301 kJ / mol。这些高效的Sn-Beta沸石可以为开发具有高稳定性的新一代PDH催化剂提供新的可能性,以生产丙烯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号