当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ene–Yne Metathesis of Allylphosphonates and Allylphosphates: Synthesis of Phosphorus-Containing 1,3-Dienes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-05 , DOI: 10.1021/acs.joc.0c02886 Laurence N. Rohde 1 , Thérèse H. Wild 1 , Steven T. Diver 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-05 , DOI: 10.1021/acs.joc.0c02886 Laurence N. Rohde 1 , Thérèse H. Wild 1 , Steven T. Diver 1
Affiliation
|
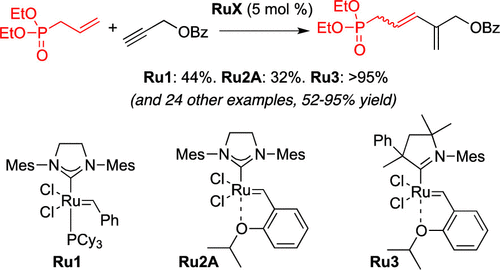
A variety of ene–yne cross metathesis reactions were performed using unsaturated phosphonate and phosphate reagents, affording the corresponding phosphorylated 1,3-diene products in good to excellent yields. These difficult ene–yne metatheses employed a Grubbs catalyst bearing a cyclic amino alkyl carbene ligand. A variety of terminal alkynes of varying substitution underwent the reaction, and different phosphorus-containing alkenes were found to give the conjugated diene products in high yields. The resulting dienes were further transformed by Horner-type Wittig reactions and a Diels–Alder cycloaddition.
中文翻译:

烯丙基膦酸酯和烯丙基磷酸的烯炔合成:含磷的1,3-二烯的合成
使用不饱和膦酸酯和磷酸盐试剂进行了多种烯-炔交叉复分解反应,从而以良好或优异的收率提供了相应的磷酸化1,3-二烯产物。这些困难的烯-炔复分解反应使用了带有环状氨基烷基卡宾配体的Grubbs催化剂。反应进行了各种取代度不同的末端炔烃的研究,发现不同的含磷烯烃可以高收率得到共轭二烯产物。生成的二烯通过霍纳型维蒂希反应和Diels-Alder环加成反应进一步转化。
更新日期:2021-01-16
中文翻译:

烯丙基膦酸酯和烯丙基磷酸的烯炔合成:含磷的1,3-二烯的合成
使用不饱和膦酸酯和磷酸盐试剂进行了多种烯-炔交叉复分解反应,从而以良好或优异的收率提供了相应的磷酸化1,3-二烯产物。这些困难的烯-炔复分解反应使用了带有环状氨基烷基卡宾配体的Grubbs催化剂。反应进行了各种取代度不同的末端炔烃的研究,发现不同的含磷烯烃可以高收率得到共轭二烯产物。生成的二烯通过霍纳型维蒂希反应和Diels-Alder环加成反应进一步转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号