当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Semiprocessive Hyperglycosylation of Adhesin by Bacterial Protein N-Glycosyltransferases
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-01-05 , DOI: 10.1021/acschembio.0c00848 Liubov Yakovlieva 1 , Carlos Ramírez-Palacios 2 , Siewert J Marrink 2 , Marthe T C Walvoort 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-01-05 , DOI: 10.1021/acschembio.0c00848 Liubov Yakovlieva 1 , Carlos Ramírez-Palacios 2 , Siewert J Marrink 2 , Marthe T C Walvoort 1
Affiliation
|
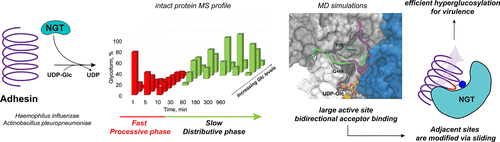
Processivity is an important feature of enzyme families such as DNA polymerases, polysaccharide synthases, and protein kinases, to ensure high fidelity in biopolymer synthesis and modification. Here, we reveal processive character in the family of cytoplasmic protein N-glycosyltransferases (NGTs). Through various activity assays, intact protein mass spectrometry, and proteomics analysis, we established that NGTs from nontypeable Haemophilus influenzae and Actinobacillus pleuropneumoniae modify an adhesin protein fragment in a semiprocessive manner. Molecular modeling studies suggest that the processivity arises from the shallow substrate binding groove in NGT, which promotes the sliding of the adhesin over the surface to allow further glycosylations without temporary dissociation. We hypothesize that the processive character of these bacterial protein glycosyltransferases is the mechanism to ensure multisite glycosylation of adhesins in vivo, thereby creating the densely glycosylated proteins necessary for bacterial self-aggregation and adherence to human cells, as a first step toward infection.
中文翻译:

细菌蛋白N-糖基转移酶对粘附素的半过程性高糖基化
加工性是酶家族(如DNA聚合酶,多糖合酶和蛋白激酶)的重要特征,以确保生物聚合物合成和修饰的高保真度。在这里,我们揭示了胞质蛋白N-糖基转移酶(NGTs)家族中的进行性特征。通过各种活性测定,完整蛋白质质谱和蛋白质组学分析,我们确定了来自不可分型流感嗜血杆菌和胸膜肺炎放线杆菌的NGT以半加工方式修饰粘附素蛋白片段。分子模型研究表明,合成能力是由NGT中较浅的底物结合槽引起的,该结合槽促进粘附素在表面上的滑动,从而允许进一步的糖基化而不会暂时解离。我们假设这些细菌蛋白糖基转移酶的加工特性是确保体内粘附素的多位糖基化的机制,从而创造出细菌自我聚集和粘附于人细胞所必需的致密糖基化蛋白,这是迈向感染的第一步。
更新日期:2021-01-15
中文翻译:

细菌蛋白N-糖基转移酶对粘附素的半过程性高糖基化
加工性是酶家族(如DNA聚合酶,多糖合酶和蛋白激酶)的重要特征,以确保生物聚合物合成和修饰的高保真度。在这里,我们揭示了胞质蛋白N-糖基转移酶(NGTs)家族中的进行性特征。通过各种活性测定,完整蛋白质质谱和蛋白质组学分析,我们确定了来自不可分型流感嗜血杆菌和胸膜肺炎放线杆菌的NGT以半加工方式修饰粘附素蛋白片段。分子模型研究表明,合成能力是由NGT中较浅的底物结合槽引起的,该结合槽促进粘附素在表面上的滑动,从而允许进一步的糖基化而不会暂时解离。我们假设这些细菌蛋白糖基转移酶的加工特性是确保体内粘附素的多位糖基化的机制,从而创造出细菌自我聚集和粘附于人细胞所必需的致密糖基化蛋白,这是迈向感染的第一步。









































 京公网安备 11010802027423号
京公网安备 11010802027423号