当前位置:
X-MOL 学术
›
J. Leukoc. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective killing of human M1 macrophages by Smac mimetics alone and M2 macrophages by Smac mimetics and caspase inhibition
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2021-01-06 , DOI: 10.1002/jlb.4a0220-114rr Hamza Ali 1, 2 , Ramon Caballero 2 , Simon X M Dong 2 , Niranjala Gajnayaka 2 , Agatha Vranjkovic 3 , Duale Ahmed 4 , Salma Iqbal 2 , Angela M Crawley 1, 3, 4, 5 , Jonathan B Angel 1, 3, 5 , Edana Cassol 6 , Ashok Kumar 1, 2, 7
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2021-01-06 , DOI: 10.1002/jlb.4a0220-114rr Hamza Ali 1, 2 , Ramon Caballero 2 , Simon X M Dong 2 , Niranjala Gajnayaka 2 , Agatha Vranjkovic 3 , Duale Ahmed 4 , Salma Iqbal 2 , Angela M Crawley 1, 3, 4, 5 , Jonathan B Angel 1, 3, 5 , Edana Cassol 6 , Ashok Kumar 1, 2, 7
Affiliation
|
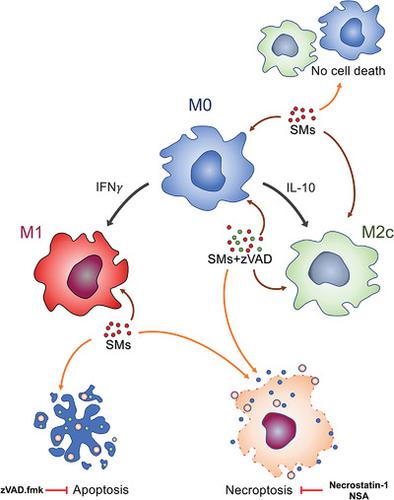
The inflammatory and anti-inflammatory Mϕs have been implicated in many diseases including rheumatoid arthritis, multiple sclerosis, and leprosy. Recent studies suggest targeting Mϕ function and activation may represent a potential target to treat these diseases. Herein, we investigated the effect of second mitochondria-derived activator of caspases (SMAC) mimetics (SMs), the inhibitors of apoptosis (IAPs) proteins, on the killing of human pro- and anti-inflammatory Mϕ subsets. We have shown previously that human monocytes are highly susceptible whereas differentiated Mϕs (M0) are highly resistant to the cytocidal abilities of SMs. To determine whether human Mϕ subsets are resistant to the cytotoxic effects of SMs, we show that M1 Mϕs are highly susceptible to SM-induced cell death whereas M2a, M2b, and M2c differentiated subsets are resistant, with M2c being the most resistant. SM-induced cell death in M1 Mϕs was mediated by apoptosis as well as necroptosis, activated both extrinsic and intrinsic pathways of apoptosis, and was attributed to the IFN-γ-mediated differentiation. In contrast, M2c and M0 Mϕs experienced cell death through necroptosis following simultaneous blockage of the IAPs and the caspase pathways. Overall, the results suggest that survival of human Mϕs is critically linked to the activation of the IAPs pathways. Moreover, agents blocking the cellular IAP1/2 and/or caspases can be exploited therapeutically to address inflammation-related diseases.
中文翻译:

单独使用 Smac 模拟物选择性杀死人 M1 巨噬细胞,以及通过 Smac 模拟物和半胱天冬酶抑制选择性杀死 M2 巨噬细胞
炎症和抗炎 Mϕ 与许多疾病有关,包括类风湿性关节炎、多发性硬化症和麻风病。最近的研究表明,靶向 Mφ 功能和激活可能代表治疗这些疾病的潜在目标。在此,我们研究了第二个线粒体衍生的半胱天冬酶 (SMAC) 模拟物 (SMs)、凋亡抑制剂 (IAPs) 蛋白对杀死人类促炎和抗炎 Mφ 亚群的影响。我们之前已经表明,人类单核细胞高度敏感,而分化的 Mϕ (M0) 对 SM 的杀细胞能力具有高度抵抗力。为了确定人类 Mφ 亚群是否对 SM 的细胞毒性作用具有抗性,我们表明 M1 Mφ 对 SM 诱导的细胞死亡高度敏感,而 M2a、M2b 和 M2c 分化亚群具有抗性,M2c 是最有抵抗力的。M1 Mφs 中 SM 诱导的细胞死亡由细胞凋亡和坏死性凋亡介导,激活细胞凋亡的外在和内在途径,并归因于 IFN-γ 介导的分化。相比之下,M2c 和 M0 Mϕs 在同时阻断 IAP 和 caspase 通路后通过坏死性凋亡经历细胞死亡。总的来说,结果表明人类 Mφs 的存活与 IAPs 通路的激活密切相关。此外,阻断细胞 IAP1/2 和/或半胱天冬酶的药物可用于治疗炎症相关疾病。并归因于 IFN-γ 介导的分化。相比之下,M2c 和 M0 Mϕs 在同时阻断 IAP 和 caspase 通路后通过坏死性凋亡经历细胞死亡。总的来说,结果表明人类 Mφs 的存活与 IAPs 通路的激活密切相关。此外,阻断细胞 IAP1/2 和/或半胱天冬酶的药物可用于治疗炎症相关疾病。并归因于 IFN-γ 介导的分化。相比之下,M2c 和 M0 Mϕs 在同时阻断 IAP 和 caspase 通路后通过坏死性凋亡经历细胞死亡。总的来说,结果表明人类 Mφs 的存活与 IAPs 通路的激活密切相关。此外,阻断细胞 IAP1/2 和/或半胱天冬酶的药物可用于治疗炎症相关疾病。
更新日期:2021-01-06
中文翻译:

单独使用 Smac 模拟物选择性杀死人 M1 巨噬细胞,以及通过 Smac 模拟物和半胱天冬酶抑制选择性杀死 M2 巨噬细胞
炎症和抗炎 Mϕ 与许多疾病有关,包括类风湿性关节炎、多发性硬化症和麻风病。最近的研究表明,靶向 Mφ 功能和激活可能代表治疗这些疾病的潜在目标。在此,我们研究了第二个线粒体衍生的半胱天冬酶 (SMAC) 模拟物 (SMs)、凋亡抑制剂 (IAPs) 蛋白对杀死人类促炎和抗炎 Mφ 亚群的影响。我们之前已经表明,人类单核细胞高度敏感,而分化的 Mϕ (M0) 对 SM 的杀细胞能力具有高度抵抗力。为了确定人类 Mφ 亚群是否对 SM 的细胞毒性作用具有抗性,我们表明 M1 Mφ 对 SM 诱导的细胞死亡高度敏感,而 M2a、M2b 和 M2c 分化亚群具有抗性,M2c 是最有抵抗力的。M1 Mφs 中 SM 诱导的细胞死亡由细胞凋亡和坏死性凋亡介导,激活细胞凋亡的外在和内在途径,并归因于 IFN-γ 介导的分化。相比之下,M2c 和 M0 Mϕs 在同时阻断 IAP 和 caspase 通路后通过坏死性凋亡经历细胞死亡。总的来说,结果表明人类 Mφs 的存活与 IAPs 通路的激活密切相关。此外,阻断细胞 IAP1/2 和/或半胱天冬酶的药物可用于治疗炎症相关疾病。并归因于 IFN-γ 介导的分化。相比之下,M2c 和 M0 Mϕs 在同时阻断 IAP 和 caspase 通路后通过坏死性凋亡经历细胞死亡。总的来说,结果表明人类 Mφs 的存活与 IAPs 通路的激活密切相关。此外,阻断细胞 IAP1/2 和/或半胱天冬酶的药物可用于治疗炎症相关疾病。并归因于 IFN-γ 介导的分化。相比之下,M2c 和 M0 Mϕs 在同时阻断 IAP 和 caspase 通路后通过坏死性凋亡经历细胞死亡。总的来说,结果表明人类 Mφs 的存活与 IAPs 通路的激活密切相关。此外,阻断细胞 IAP1/2 和/或半胱天冬酶的药物可用于治疗炎症相关疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号