当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the Halogen Bonding Strength of Cyclic Diaryliodonium Salts
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-01-06 , DOI: 10.1002/hlca.202000221 Dominik L. Reinhard 1 , Flemming Heinen 1 , Julian Stoesser 1 , Elric Engelage 1 , Stefan M. Huber 1
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-01-06 , DOI: 10.1002/hlca.202000221 Dominik L. Reinhard 1 , Flemming Heinen 1 , Julian Stoesser 1 , Elric Engelage 1 , Stefan M. Huber 1
Affiliation
|
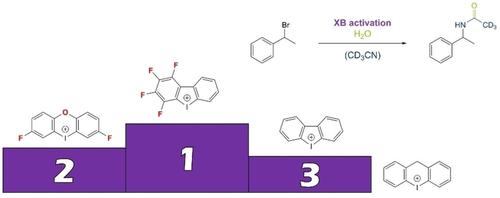
Diaryliodonium(III) salts have recently received increasing interest as a new class of strong halogen bonding noncovalent organocatalysts. Even though this utilization of their Lewis acidity has only been investigated in few studies, their high potential in comparison to classical monovalent iodine based XB donors has become very apparent. So far, only acyclic and cyclic five‐membered core structures have been used, and titration studies have shown the latter to be superior in terms of binding constants towards Lewis bases. Herein, we now compare the Lewis acidity and activity of these five‐membered iodolium salts with those of six‐membered iodininium derivatives. X‐Ray structural analyses, ITC measurements and the reaction kinetics of a Ritter‐type solvolysis reaction in wet acetonitrile (a typical halogen bond donor benchmark reaction) all demonstrate that iodolium salts are stronger halogen bond donors and catalysts than iodininium salts. Subsequently, we were able to improve the activity of both core structures significantly by introducing electron‐withdrawing substituents.
中文翻译:

调整环状二芳基碘鎓盐的卤素键强度
作为一种新型的强卤素键合非共价有机催化剂,二芳基碘鎓(III)盐最近受到越来越多的关注。尽管仅在很少的研究中对这种路易斯酸的利用进行了研究,但与基于传统单价碘的XB供体相比,它们的高潜力已变得非常明显。到目前为止,仅使用了无环和环状五元核结构,滴定研究表明,后者在与Lewis碱的结合常数方面具有优势。在这里,我们现在比较这些五元碘盐和六元碘鎓衍生物的路易斯酸度和活性。X-Ray结构分析,ITC测量和Ritter的反应动力学湿乙腈中的溶剂型反应(典型的卤素键供体基准反应)均表明,碘鎓盐比碘鎓盐是更强的卤素键供体和催化剂。随后,我们能够通过引入吸电子取代基来显着提高两个核心结构的活性。
更新日期:2021-02-12
中文翻译:

调整环状二芳基碘鎓盐的卤素键强度
作为一种新型的强卤素键合非共价有机催化剂,二芳基碘鎓(III)盐最近受到越来越多的关注。尽管仅在很少的研究中对这种路易斯酸的利用进行了研究,但与基于传统单价碘的XB供体相比,它们的高潜力已变得非常明显。到目前为止,仅使用了无环和环状五元核结构,滴定研究表明,后者在与Lewis碱的结合常数方面具有优势。在这里,我们现在比较这些五元碘盐和六元碘鎓衍生物的路易斯酸度和活性。X-Ray结构分析,ITC测量和Ritter的反应动力学湿乙腈中的溶剂型反应(典型的卤素键供体基准反应)均表明,碘鎓盐比碘鎓盐是更强的卤素键供体和催化剂。随后,我们能够通过引入吸电子取代基来显着提高两个核心结构的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号