当前位置:
X-MOL 学术
›
J. Mol. Recognit.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the structural basis and atomistic binding mechanistic of the selective antagonist blockade at D3 dopamine receptor over D2 dopamine receptor
Journal of Molecular Recognition ( IF 2.3 ) Pub Date : 2021-01-05 , DOI: 10.1002/jmr.2885 Patrick Appiah-Kubi 1 , Fisayo Andrew Olotu 1 , Mahmoud E S Soliman 1
Journal of Molecular Recognition ( IF 2.3 ) Pub Date : 2021-01-05 , DOI: 10.1002/jmr.2885 Patrick Appiah-Kubi 1 , Fisayo Andrew Olotu 1 , Mahmoud E S Soliman 1
Affiliation
|
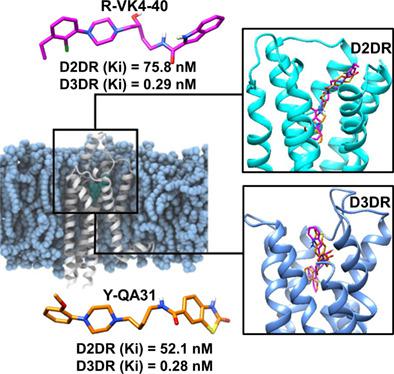
More recently, there has been a paradigm shift toward selective drug targeting in the treatment of neurological disorders, including drug addiction, schizophrenia, and Parkinson's disease mediated by the different dopamine receptor subtypes. Antagonists with higher selectivity for D3 dopamine receptor (D3DR) over D2 dopamine receptor (D2DR) have been shown to attenuate drug‐seeking behavior and associated side effects compared to non‐subtype selective antagonists. However, high conservations among constituent residues of both proteins, particularly at the ligand‐binding pockets, remain a challenge to therapeutic drug design. Recent studies have reported the discovery of two small‐molecules R‐VK4‐40 and Y‐QA31 which substantially inhibited D3DR with >180‐fold selectivity over D2DR. Therefore, in this study, we seek to provide molecular and structural insights into these differential binding mechanistic using meta‐analytic computational simulation methods. Findings revealed that R‐VK4‐40 and Y‐QA31 adopted shallow binding modes and were more surface‐exposed at D3DR while on the contrary, they exhibited deep hydrophobic pocket binding at D2DR. Also, two non‐conserved residues; Tyr361.39 and Ser18245.51 were identified in D3DR, based on their crucial roles and contributions to the selective binding of R‐VK4‐40 and Y‐QA31. Importantly, both antagonists exhibited high affinities in complex with D3DR compared to D2DR, while van der Waals energies contributed majorly to their binding and stability. Structural analyses also revealed the distinct stabilizing effects of both compounds on D3DR secondary architecture relative to D2DR. Therefore, findings herein pinpointed the origin and mechanistic of selectivity of the compounds, which may assist in the rational design of potential small molecules of the D2‐like dopamine family receptor subtype with improved potency and selectivity.
中文翻译:

探索选择性拮抗剂阻断 D3 多巴胺受体优于 D2 多巴胺受体的结构基础和原子结合机制
最近,在治疗神经系统疾病(包括药物成瘾、精神分裂症和由不同多巴胺受体亚型介导的帕金森病)中出现了选择性药物靶向治疗的范式转变。对 D 3多巴胺受体 (D3DR) 的选择性高于 D 2的拮抗剂与非亚型选择性拮抗剂相比,多巴胺受体 (D2DR) 已被证明可以减轻寻求药物的行为和相关的副作用。然而,两种蛋白质的组成残基之间的高度保守性,特别是在配体结合口袋中,仍然是治疗药物设计的挑战。最近的研究报道了两种小分子 R-VK4-40 和 Y-QA31 的发现,它们显着抑制 D3DR,其选择性是 D2DR 的 180 倍以上。因此,在这项研究中,我们寻求使用元分析计算模拟方法为这些差异结合机制提供分子和结构方面的见解。研究结果表明,R-VK4-40 和 Y-QA31 采用浅结合模式,在 D3DR 时表面暴露更多,相反,它们在 D2DR 时表现出深疏水袋结合。还,两个非保守残基;酪氨酸36基于它们对 R-VK4-40 和 Y-QA31 选择性结合的关键作用和贡献,在 D3DR 中鉴定了1.39和 Ser182 45.51 。重要的是,与 D2DR 相比,两种拮抗剂与 D3DR 的复合物表现出高亲和力,而范德华能量主要有助于它们的结合和稳定性。结构分析还揭示了两种化合物相对于 D2DR 对 D3DR 二级结构的明显稳定作用。因此,本文的研究结果确定了化合物选择性的来源和机制,这可能有助于合理设计具有改善效力和选择性的 D 2样多巴胺家族受体亚型的潜在小分子。
更新日期:2021-01-05
中文翻译:

探索选择性拮抗剂阻断 D3 多巴胺受体优于 D2 多巴胺受体的结构基础和原子结合机制
最近,在治疗神经系统疾病(包括药物成瘾、精神分裂症和由不同多巴胺受体亚型介导的帕金森病)中出现了选择性药物靶向治疗的范式转变。对 D 3多巴胺受体 (D3DR) 的选择性高于 D 2的拮抗剂与非亚型选择性拮抗剂相比,多巴胺受体 (D2DR) 已被证明可以减轻寻求药物的行为和相关的副作用。然而,两种蛋白质的组成残基之间的高度保守性,特别是在配体结合口袋中,仍然是治疗药物设计的挑战。最近的研究报道了两种小分子 R-VK4-40 和 Y-QA31 的发现,它们显着抑制 D3DR,其选择性是 D2DR 的 180 倍以上。因此,在这项研究中,我们寻求使用元分析计算模拟方法为这些差异结合机制提供分子和结构方面的见解。研究结果表明,R-VK4-40 和 Y-QA31 采用浅结合模式,在 D3DR 时表面暴露更多,相反,它们在 D2DR 时表现出深疏水袋结合。还,两个非保守残基;酪氨酸36基于它们对 R-VK4-40 和 Y-QA31 选择性结合的关键作用和贡献,在 D3DR 中鉴定了1.39和 Ser182 45.51 。重要的是,与 D2DR 相比,两种拮抗剂与 D3DR 的复合物表现出高亲和力,而范德华能量主要有助于它们的结合和稳定性。结构分析还揭示了两种化合物相对于 D2DR 对 D3DR 二级结构的明显稳定作用。因此,本文的研究结果确定了化合物选择性的来源和机制,这可能有助于合理设计具有改善效力和选择性的 D 2样多巴胺家族受体亚型的潜在小分子。










































 京公网安备 11010802027423号
京公网安备 11010802027423号