Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2021-01-06 , DOI: 10.1016/j.bbamem.2020.183548 Muhasin Koyiloth 1 , Sathyanarayana N Gummadi 1
|
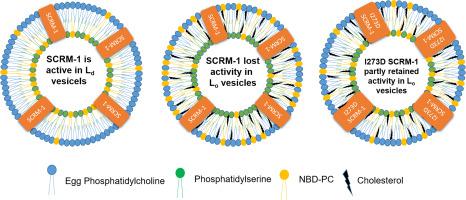
Phospholipid (PL) scramblases are single-pass transmembrane protein mediating bidirectional PL translocation. Previously in silico analysis of human PL scramblases, predicted the presence of an uncharacterized cholesterol-binding domain spanning partly in the transmembrane helix as well as in the adjacent extracellular coil. This domain was found to be universally conserved in diverse organisms like Caenorhabditis elegans. In this study, we investigated the saturable cholesterol-binding domain of SCRM-1 using fluorescence sterol binding assay, Stern-Volmer quenching, Förster resonance energy transfer, and CD spectroscopy. We observed high-affinity interaction between cholesterol and SCRM-1. Our results support a previous report, which showed that the cholesterol ordering effect reduced the scramblase activity of hPLSCR1. Considering the presence of a high-affinity binding sequence, we propose that the reduction in activity could partly be due to the cholesterol binding. To validate this, we generated a C-terminal helix (CTH) deletion construct (∆CTH SCRM-1) and a point mutation in the putative cholesterol-binding domain I273D SCRM-1. Deletion construct greatly reduced cholesterol affinity along with loss of scramblase activity. In contrast to this, I273D SCRM-1 retained scrambling activity in proteoliposomes containing ~30 mol% cholesterol but lost sterol binding ability. These results suggest that C-terminal helix is crucial for membrane insertion and in the lipid bilayer the scrambling activity of SCRM-1 is modulated through its interaction with cholesterol.
中文翻译:

胆固醇相互作用减弱了人造膜中 SCRM-1 的加扰酶活性
磷脂 (PL) 乱序是单程跨膜蛋白,介导双向 PL 易位。以前在人类 PL 乱序的计算机分析中,预测了部分跨膜螺旋以及相邻的细胞外螺旋中存在未表征的胆固醇结合域。发现该结构域在不同生物体中普遍保守,如秀丽隐杆线虫. 在这项研究中,我们使用荧光甾醇结合测定、Stern-Volmer 淬灭、Förster 共振能量转移和 CD 光谱研究了 SCRM-1 的可饱和胆固醇结合域。我们观察到胆固醇和 SCRM-1 之间的高亲和力相互作用。我们的结果支持之前的报告,该报告表明胆固醇排序效应降低了 hPLSCR1 的加扰酶活性。考虑到高亲和力结合序列的存在,我们认为活性降低可能部分是由于胆固醇结合。为了验证这一点,我们在假定的胆固醇结合域 I273D SCRM-1 中生成了一个 C 端螺旋 (CTH) 缺失构建体 (ΔCTH SCRM-1) 和点突变。删除构建体大大降低了胆固醇亲和力以及加扰酶活性的丧失。与此相反,I273D SCRM-1 在含有约 30 mol% 胆固醇的蛋白脂质体中保留了加扰活性,但失去了甾醇结合能力。这些结果表明 C 端螺旋对于膜插入至关重要,并且在脂质双层中,SCRM-1 的加扰活性通过其与胆固醇的相互作用进行调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号