当前位置:
X-MOL 学术
›
J. Anal. At. Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
XAS study of Sn speciation in toothpaste
Journal of Analytical Atomic Spectrometry ( IF 3.4 ) Pub Date : 2020-12-17 , DOI: 10.1039/d0ja00392a Morgane Desmau 1, 2, 3, 4 , Marco A. Alsina 5, 6, 7, 8 , Jean-François Gaillard 1, 2, 3, 4
Journal of Analytical Atomic Spectrometry ( IF 3.4 ) Pub Date : 2020-12-17 , DOI: 10.1039/d0ja00392a Morgane Desmau 1, 2, 3, 4 , Marco A. Alsina 5, 6, 7, 8 , Jean-François Gaillard 1, 2, 3, 4
Affiliation
|
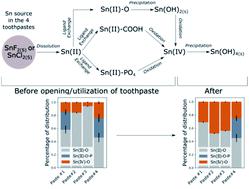
Stannous fluoride (SnF2) is an effective antimicrobial agent and fluoride carrier to dental enamel. However, stannous complexes are known to readily oxidize in presence of O2 and interact with a variety of ligands such as organic and inorganic compounds commonly present in toothpaste. Therefore, the stability of stannous species inside toothpaste remains an open question. Here we probe the speciation of Sn in solutions of known composition and in commercial toothpaste using Sn K-edge X-ray absorption spectroscopy. We show that the oxidation state of Sn and its chemical nature depends on both paste composition and time of opening, with Sn ultimately oxidizing into amorphous Sn(IV) hydroxide, Sn(OH)4. These results highlight the effect of paste composition and utilization on the speciation of active ingredients such as SnF2, and contribute to a rational design of toothpaste formulations that enhances the stability of stannous agents.
中文翻译:

XAS研究牙膏中锡的形态
氟化亚锡(SnF 2)是一种有效的抗菌剂,也是牙釉质的氟化物载体。然而,已知亚锡配合物在O 2存在下容易氧化并与多种配体相互作用,例如牙膏中通常存在的有机和无机化合物。因此,牙膏中亚锡物质的稳定性仍然是一个悬而未决的问题。在这里,我们使用Sn K边缘X射线吸收光谱法研究已知成分的溶液和商用牙膏中Sn的形态。我们表明,锡的氧化态及其化学性质取决于糊剂的成分和打开时间,锡最终会氧化成非晶态的Sn(IV)氢氧化物Sn(OH)4。这些结果突出了糊剂组成和利用率对活性成分如SnF 2的形成的影响,并有助于合理设计可增强亚锡试剂稳定性的牙膏配方。
更新日期:2021-01-05
中文翻译:

XAS研究牙膏中锡的形态
氟化亚锡(SnF 2)是一种有效的抗菌剂,也是牙釉质的氟化物载体。然而,已知亚锡配合物在O 2存在下容易氧化并与多种配体相互作用,例如牙膏中通常存在的有机和无机化合物。因此,牙膏中亚锡物质的稳定性仍然是一个悬而未决的问题。在这里,我们使用Sn K边缘X射线吸收光谱法研究已知成分的溶液和商用牙膏中Sn的形态。我们表明,锡的氧化态及其化学性质取决于糊剂的成分和打开时间,锡最终会氧化成非晶态的Sn(IV)氢氧化物Sn(OH)4。这些结果突出了糊剂组成和利用率对活性成分如SnF 2的形成的影响,并有助于合理设计可增强亚锡试剂稳定性的牙膏配方。



























 京公网安备 11010802027423号
京公网安备 11010802027423号