当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent progress in electrochemical synthesis of ammonia from nitrogen: strategies to improve the catalytic activity and selectivity
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0ee02263b Bing Yang 1, 2, 3, 4, 5 , Weilu Ding 1, 2, 3, 4, 5 , Honghua Zhang 1, 2, 3, 4, 5 , Suojiang Zhang 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0ee02263b Bing Yang 1, 2, 3, 4, 5 , Weilu Ding 1, 2, 3, 4, 5 , Honghua Zhang 1, 2, 3, 4, 5 , Suojiang Zhang 1, 2, 3, 4, 5
Affiliation
|
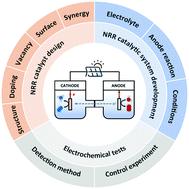
The modern ammonia synthesis industry founded by Haber–Bosch in 1913 has successfully altered the history of food production, fed explosive population growth, and laid the foundation of heterogeneous catalysis and chemical engineering as well. However, its reliance on fossil fuels for reactant H2 production consumes 1–3% of the world's electric energy and 2–5% of the world's natural gas, accompanied by more than 400 million tons of CO2 emission annually. Making use of water as the proton source and electric energy to drive the ammonia synthesis reaction will reduce the fossil fuel consumption and CO2 emission and is thus regarded as a green and sustainable alternative to the conventional Haber–Bosch process. To date, some excellent reviews on electrochemical ammonia synthesis have been published, but most of them were organized according to the type of catalyst. A systematic summary of the performance-improving strategies of the electrocatalyst for ammonia synthesis is rarely reported and therefore highly desirable. In this review, the nitrogen reduction reaction mechanisms and recent theoretical advances are briefly outlined first. Then, strategies for both reactivity and selectivity enhancement of catalysts and catalytic systems are methodically discussed. Last, criteria for the electrochemical nitrogen reduction reaction, ammonia quantification methods, and an outlook for further research are also concluded. This review aims to provide systematic and concise guidance for the design of highly efficient and selective electrochemical ammonia synthesis systems.
中文翻译:

氮电化学合成氨的最新进展:提高催化活性和选择性的策略
Haber–Bosch于1913年创立的现代氨合成工业成功地改变了食品生产的历史,为爆炸性的人口增长提供了动力,并奠定了多相催化和化学工程的基础。然而,其依靠化石燃料生产H 2的反应物消耗了全球电能的1-3%和天然气的2%至5%,每年排放的CO 2超过4亿吨。利用水作为质子源和电能驱动氨合成反应将减少化石燃料的消耗和CO 2排放,因此被视为常规Haber-Bosch工艺的绿色和可持续替代方案。迄今为止,已经发表了一些关于电化学合成氨的出色综述,但其中大多数是根据催化剂的类型进行组织的。关于氨合成的电催化剂的性能改进策略的系统总结很少报道,因此非常需要。在这篇综述中,首先简要概述了氮还原反应机理和最新的理论进展。然后,系统地讨论了催化剂和催化体系的反应性和选择性增强的策略。最后,还总结了电化学氮还原反应的标准,氨的定量方法以及进一步研究的前景。
更新日期:2021-01-05
中文翻译:

氮电化学合成氨的最新进展:提高催化活性和选择性的策略
Haber–Bosch于1913年创立的现代氨合成工业成功地改变了食品生产的历史,为爆炸性的人口增长提供了动力,并奠定了多相催化和化学工程的基础。然而,其依靠化石燃料生产H 2的反应物消耗了全球电能的1-3%和天然气的2%至5%,每年排放的CO 2超过4亿吨。利用水作为质子源和电能驱动氨合成反应将减少化石燃料的消耗和CO 2排放,因此被视为常规Haber-Bosch工艺的绿色和可持续替代方案。迄今为止,已经发表了一些关于电化学合成氨的出色综述,但其中大多数是根据催化剂的类型进行组织的。关于氨合成的电催化剂的性能改进策略的系统总结很少报道,因此非常需要。在这篇综述中,首先简要概述了氮还原反应机理和最新的理论进展。然后,系统地讨论了催化剂和催化体系的反应性和选择性增强的策略。最后,还总结了电化学氮还原反应的标准,氨的定量方法以及进一步研究的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号