当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
HnRNPA2B1 promotes the proliferation of breast cancer MCF‐7 cells via the STAT3 pathway
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2021-01-05 , DOI: 10.1002/jcb.29875 Li-Bin Gao 1, 2 , Xin-Le Zhu 1 , Jing-Xian Shi 1 , Ling Yang 1 , Zhen-Qiang Xu 3 , Song-Lin Shi 1
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2021-01-05 , DOI: 10.1002/jcb.29875 Li-Bin Gao 1, 2 , Xin-Le Zhu 1 , Jing-Xian Shi 1 , Ling Yang 1 , Zhen-Qiang Xu 3 , Song-Lin Shi 1
Affiliation
|
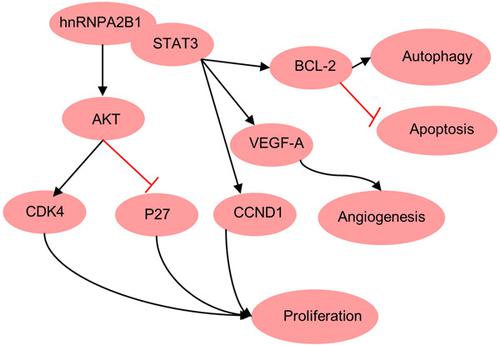
HnRNPA2/B1 is highly expressed in many tumors. However, the role of hnRNPA2/B1 in breast cancer is not clear. In this study, we found the proliferation rate was decreased after knockout of hnRNPA2/B1 by CRISPR‐CAS9 in MCF‐7 cells, as demonstrated by the reduced expression of CDK4 and p‐AKT, and the increased expression of P27. Besides this, the western blot results showed that knockout of hnRNPA2/B1 increased the rate of apoptosis and declined autophagy. By in vivo assay, we found that knockout of hnRNPA2/B1 suppressed tumor growth in a xenograft mouse model. Immunohistochemical staining results confirmed knockout of hnRNPA2/B1 impaired tumor angiogenesis, as illustrated by downregulated expression of VEGF‐A. Besides this, interacting proteins with hnRNPA2/B1 were identified by mass spectrometry and the PPI network was constructed. GO analysis suggests that the Interacting proteins are mainly enriched in the Wnt signaling pathway, tumor necrosis factor‐mediated signaling pathway, translation, and so on. We then identified hnRNPA2/B1 interacted with signal transducer and activator of transcription 3 (STAT3), as supported by the colocalization of hnRNPA2/B1 and STAT3. Meanwhile, knockout of hnRNPA2/B1 inhibited the phosphorylation of STAT3. Collectively, our results demonstrate that hnRNPA2/B1 promotes tumor cell growth in vitro and in vivo by activating the STAT3 pathway, regulating apoptosis and autophagy.
中文翻译:

HnRNPA2B1通过STAT3通路促进乳腺癌MCF-7细胞增殖
HnRNPA2/B1 在许多肿瘤中高度表达。然而,hnRNPA2/B1 在乳腺癌中的作用尚不清楚。在本研究中,我们发现 MCF-7 细胞中 CRISPR-CAS9 敲除 hnRNPA2/B1 后增殖率降低,这表现为 CDK4 和 p-AKT 表达降低以及 P27 表达增加。除此之外,蛋白质印迹结果表明,hnRNPA2/B1 的敲除增加了细胞凋亡率并降低了自噬。通过体内试验,我们发现 hnRNPA2/B1 的敲除抑制了异种移植小鼠模型中的肿瘤生长。免疫组织化学染色结果证实了 hnRNPA2/B1 的敲除会损害肿瘤血管生成,如 VEGF-A 的下调表达所示。除此之外,通过质谱鉴定与hnRNPA2/B1相互作用的蛋白质并构建了PPI网络。GO分析表明相互作用蛋白主要富集在Wnt信号通路、肿瘤坏死因子介导的信号通路、翻译等。然后我们确定了 hnRNPA2/B1 与信号转导和转录激活因子 3 (STAT3) 相互作用,这得到 hnRNPA2/B1 和 STAT3 的共定位的支持。同时,hnRNPA2/B1 的敲除抑制了 STAT3 的磷酸化。总的来说,我们的结果表明 hnRNPA2/B1 通过激活 STAT3 通路、调节细胞凋亡和自噬来促进体外和体内肿瘤细胞的生长。由 hnRNPA2/B1 和 STAT3 的共定位支持。同时,hnRNPA2/B1 的敲除抑制了 STAT3 的磷酸化。总的来说,我们的结果表明 hnRNPA2/B1 通过激活 STAT3 通路、调节细胞凋亡和自噬来促进体外和体内肿瘤细胞的生长。由 hnRNPA2/B1 和 STAT3 的共定位支持。同时,hnRNPA2/B1 的敲除抑制了 STAT3 的磷酸化。总的来说,我们的结果表明 hnRNPA2/B1 通过激活 STAT3 通路、调节细胞凋亡和自噬来促进体外和体内肿瘤细胞的生长。
更新日期:2021-02-16
中文翻译:

HnRNPA2B1通过STAT3通路促进乳腺癌MCF-7细胞增殖
HnRNPA2/B1 在许多肿瘤中高度表达。然而,hnRNPA2/B1 在乳腺癌中的作用尚不清楚。在本研究中,我们发现 MCF-7 细胞中 CRISPR-CAS9 敲除 hnRNPA2/B1 后增殖率降低,这表现为 CDK4 和 p-AKT 表达降低以及 P27 表达增加。除此之外,蛋白质印迹结果表明,hnRNPA2/B1 的敲除增加了细胞凋亡率并降低了自噬。通过体内试验,我们发现 hnRNPA2/B1 的敲除抑制了异种移植小鼠模型中的肿瘤生长。免疫组织化学染色结果证实了 hnRNPA2/B1 的敲除会损害肿瘤血管生成,如 VEGF-A 的下调表达所示。除此之外,通过质谱鉴定与hnRNPA2/B1相互作用的蛋白质并构建了PPI网络。GO分析表明相互作用蛋白主要富集在Wnt信号通路、肿瘤坏死因子介导的信号通路、翻译等。然后我们确定了 hnRNPA2/B1 与信号转导和转录激活因子 3 (STAT3) 相互作用,这得到 hnRNPA2/B1 和 STAT3 的共定位的支持。同时,hnRNPA2/B1 的敲除抑制了 STAT3 的磷酸化。总的来说,我们的结果表明 hnRNPA2/B1 通过激活 STAT3 通路、调节细胞凋亡和自噬来促进体外和体内肿瘤细胞的生长。由 hnRNPA2/B1 和 STAT3 的共定位支持。同时,hnRNPA2/B1 的敲除抑制了 STAT3 的磷酸化。总的来说,我们的结果表明 hnRNPA2/B1 通过激活 STAT3 通路、调节细胞凋亡和自噬来促进体外和体内肿瘤细胞的生长。由 hnRNPA2/B1 和 STAT3 的共定位支持。同时,hnRNPA2/B1 的敲除抑制了 STAT3 的磷酸化。总的来说,我们的结果表明 hnRNPA2/B1 通过激活 STAT3 通路、调节细胞凋亡和自噬来促进体外和体内肿瘤细胞的生长。










































 京公网安备 11010802027423号
京公网安备 11010802027423号