当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Umbilical cord mesenchymal stem cells for COVID‐19 acute respiratory distress syndrome: A double‐blind, phase 1/2a, randomized controlled trial
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2021-01-05 , DOI: 10.1002/sctm.20-0472 Giacomo Lanzoni 1, 2 , Elina Linetsky 1, 3 , Diego Correa 1, 4 , Shari Messinger Cayetano 5 , Roger A Alvarez 6, 7 , Dimitrios Kouroupis 1 , Ana Alvarez Gil 1 , Raffaella Poggioli 1 , Phillip Ruiz 3 , Antonio C Marttos 6, 7, 8 , Khemraj Hirani 1, 6 , Crystal A Bell 6 , Halina Kusack 6 , Lisa Rafkin 1 , David Baidal 1, 6, 7 , Andrew Pastewski 8 , Kunal Gawri 6, 7 , Clarissa Leñero 1 , Alejandro M A Mantero 5 , Sarah W Metalonis 5 , Xiaojing Wang 1 , Luis Roque 1 , Burlett Masters 1 , Norma S Kenyon 1 , Enrique Ginzburg 3, 7, 8 , Xiumin Xu 1 , Jianming Tan 9 , Arnold I Caplan 10 , Marilyn K Glassberg 11 , Rodolfo Alejandro 1, 6, 7 , Camillo Ricordi 1, 3
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2021-01-05 , DOI: 10.1002/sctm.20-0472 Giacomo Lanzoni 1, 2 , Elina Linetsky 1, 3 , Diego Correa 1, 4 , Shari Messinger Cayetano 5 , Roger A Alvarez 6, 7 , Dimitrios Kouroupis 1 , Ana Alvarez Gil 1 , Raffaella Poggioli 1 , Phillip Ruiz 3 , Antonio C Marttos 6, 7, 8 , Khemraj Hirani 1, 6 , Crystal A Bell 6 , Halina Kusack 6 , Lisa Rafkin 1 , David Baidal 1, 6, 7 , Andrew Pastewski 8 , Kunal Gawri 6, 7 , Clarissa Leñero 1 , Alejandro M A Mantero 5 , Sarah W Metalonis 5 , Xiaojing Wang 1 , Luis Roque 1 , Burlett Masters 1 , Norma S Kenyon 1 , Enrique Ginzburg 3, 7, 8 , Xiumin Xu 1 , Jianming Tan 9 , Arnold I Caplan 10 , Marilyn K Glassberg 11 , Rodolfo Alejandro 1, 6, 7 , Camillo Ricordi 1, 3
Affiliation
|
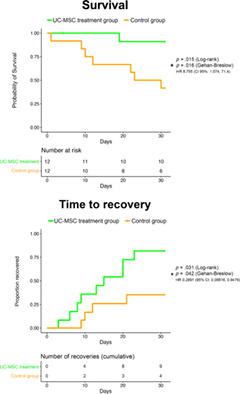
Acute respiratory distress syndrome (ARDS) in COVID‐19 is associated with high mortality. Mesenchymal stem cells are known to exert immunomodulatory and anti‐inflammatory effects and could yield beneficial effects in COVID‐19 ARDS. The objective of this study was to determine safety and explore efficacy of umbilical cord mesenchymal stem cell (UC‐MSC) infusions in subjects with COVID‐19 ARDS. A double‐blind, phase 1/2a, randomized, controlled trial was performed. Randomization and stratification by ARDS severity was used to foster balance among groups. All subjects were analyzed under intention to treat design. Twenty‐four subjects were randomized 1:1 to either UC‐MSC treatment (n = 12) or the control group (n = 12). Subjects in the UC‐MSC treatment group received two intravenous infusions (at day 0 and 3) of 100 ± 20 × 106 UC‐MSCs; controls received two infusions of vehicle solution. Both groups received best standard of care. Primary endpoint was safety (adverse events [AEs]) within 6 hours; cardiac arrest or death within 24 hours postinfusion). Secondary endpoints included patient survival at 31 days after the first infusion and time to recovery. No difference was observed between groups in infusion‐associated AEs. No serious adverse events (SAEs) were observed related to UC‐MSC infusions. UC‐MSC infusions in COVID‐19 ARDS were found to be safe. Inflammatory cytokines were significantly decreased in UC‐MSC‐treated subjects at day 6. Treatment was associated with significantly improved patient survival (91% vs 42%, P = .015), SAE‐free survival (P = .008), and time to recovery (P = .03). UC‐MSC infusions are safe and could be beneficial in treating subjects with COVID‐19 ARDS.
中文翻译:

脐带间充质干细胞治疗 COVID-19 急性呼吸窘迫综合征:双盲、1/2a 期随机对照试验
COVID-19 中的急性呼吸窘迫综合征 (ARDS) 与高死亡率相关。众所周知,间充质干细胞具有免疫调节和抗炎作用,可能对 COVID-19 ARDS 产生有益作用。本研究的目的是确定 COVID-19 ARDS 受试者输注脐带间充质干细胞 (UC-MSC) 的安全性并探讨其疗效。进行了一项双盲 1/2a 期随机对照试验。根据 ARDS 严重程度进行随机化和分层,以促进各组之间的平衡。所有受试者均根据意向治疗设计进行分析。 24 名受试者以 1:1 的比例随机分配至 UC-MSC 治疗组 (n = 12) 或对照组 (n = 12)。 UC-MSC 治疗组的受试者接受两次静脉输注(第 0 天和第 3 天)100 ± 20 × 10 6 UC-MSC;对照接受两次载体溶液输注。两组均接受了最佳标准的护理。主要终点是 6 小时内的安全性(不良事件 [AE]);输注后 24 小时内心脏骤停或死亡)。次要终点包括首次输注后 31 天的患者生存率和恢复时间。在输注相关 AE 方面,各组之间没有观察到差异。未观察到与 UC-MSC 输注相关的严重不良事件 (SAE)。研究发现 UC-MSC 输注治疗 COVID-19 ARDS 是安全的。第 6 天,接受 UC-MSC 治疗的受试者的炎症细胞因子显着降低。治疗与患者生存率(91% vs 42%, P = .015)、无 SAE 生存率( P = .008)和时间的显着改善相关。恢复 ( P = .03)。 UC-MSC 输注是安全的,并且可能有益于治疗 COVID-19 ARDS 受试者。
更新日期:2021-01-05
中文翻译:

脐带间充质干细胞治疗 COVID-19 急性呼吸窘迫综合征:双盲、1/2a 期随机对照试验
COVID-19 中的急性呼吸窘迫综合征 (ARDS) 与高死亡率相关。众所周知,间充质干细胞具有免疫调节和抗炎作用,可能对 COVID-19 ARDS 产生有益作用。本研究的目的是确定 COVID-19 ARDS 受试者输注脐带间充质干细胞 (UC-MSC) 的安全性并探讨其疗效。进行了一项双盲 1/2a 期随机对照试验。根据 ARDS 严重程度进行随机化和分层,以促进各组之间的平衡。所有受试者均根据意向治疗设计进行分析。 24 名受试者以 1:1 的比例随机分配至 UC-MSC 治疗组 (n = 12) 或对照组 (n = 12)。 UC-MSC 治疗组的受试者接受两次静脉输注(第 0 天和第 3 天)100 ± 20 × 10 6 UC-MSC;对照接受两次载体溶液输注。两组均接受了最佳标准的护理。主要终点是 6 小时内的安全性(不良事件 [AE]);输注后 24 小时内心脏骤停或死亡)。次要终点包括首次输注后 31 天的患者生存率和恢复时间。在输注相关 AE 方面,各组之间没有观察到差异。未观察到与 UC-MSC 输注相关的严重不良事件 (SAE)。研究发现 UC-MSC 输注治疗 COVID-19 ARDS 是安全的。第 6 天,接受 UC-MSC 治疗的受试者的炎症细胞因子显着降低。治疗与患者生存率(91% vs 42%, P = .015)、无 SAE 生存率( P = .008)和时间的显着改善相关。恢复 ( P = .03)。 UC-MSC 输注是安全的,并且可能有益于治疗 COVID-19 ARDS 受试者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号