当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational investigation of the substituent effect in the [2 + 4] Diels–Alder cycloaddition reactions of HSi≡Si(para-C6H4X) with benzene
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2021-01-05 , DOI: 10.1002/jccs.202000428 Mina Ahraminejad 1 , Reza Ghiasi 2 , Bita Mohtat 1 , Roya Ahmadi 3
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2021-01-05 , DOI: 10.1002/jccs.202000428 Mina Ahraminejad 1 , Reza Ghiasi 2 , Bita Mohtat 1 , Roya Ahmadi 3
Affiliation
|
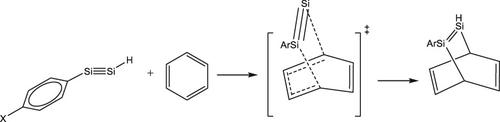
In this paper, the DFT method was applied at the B3LYP/6–311+G (d,p) level of theory to investigate the [2 + 4] Diels−Alder cycloaddition reactions of HSi≡Si(para-C6H4X); X = NH2, OH, Me, H, F, CCH, OCF3 with benzene. It was attempted to show how the substituent affects the barrier height and thermodynamic parameters of these reactions. To illustrate the interaction between two fragments at transition states Activation Strain Model (ASM) and Energy Decomposition Analysis (EDA) were utilized. The Wiberg bond indices were used to check the progress of the reactions. The rate constant of the reaction was computed at 300 K, in the gas phase. Also, the synchronicity values of this reaction were determined.
中文翻译:

HSi≡Si(对-C6H4X)与苯的[2 + 4] Diels-Alder环加成反应中取代基效应的计算研究
在本文中,在DFT方法在理论的B3LYP / 6-311 + G(d,p)水平施加到调查[2 + 4]狄尔斯-阿尔德在HSi环加成反应≡的Si(对-C 6 H ^ 4 X); X = NH 2,OH,Me,H,F,CCH,OCF 3与苯。试图表明取代基如何影响这些反应的势垒高度和热力学参数。为了说明两个片段在过渡态之间的相互作用,使用了活化应变模型(ASM)和能量分解分析(EDA)。Wiberg键指数用于检查反应进程。在气相中,在300K下计算反应的速率常数。同样,确定该反应的同步性值。
更新日期:2021-01-05
中文翻译:

HSi≡Si(对-C6H4X)与苯的[2 + 4] Diels-Alder环加成反应中取代基效应的计算研究
在本文中,在DFT方法在理论的B3LYP / 6-311 + G(d,p)水平施加到调查[2 + 4]狄尔斯-阿尔德在HSi环加成反应≡的Si(对-C 6 H ^ 4 X); X = NH 2,OH,Me,H,F,CCH,OCF 3与苯。试图表明取代基如何影响这些反应的势垒高度和热力学参数。为了说明两个片段在过渡态之间的相互作用,使用了活化应变模型(ASM)和能量分解分析(EDA)。Wiberg键指数用于检查反应进程。在气相中,在300K下计算反应的速率常数。同样,确定该反应的同步性值。









































 京公网安备 11010802027423号
京公网安备 11010802027423号