当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IL‐37‐induced activation of glycogen synthase kinase 3β promotes IL‐1R8/Sigirr phosphorylation, internalization, and degradation in lung epithelial cells
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2021-01-05 , DOI: 10.1002/jcp.30253 Lian Li 1 , Jianxin Wei 2 , Tomeka L Suber 2 , Qinmao Ye 1 , Jiaxing Miao 1 , Shuang Li 2 , Sarah J Taleb 1 , Kevin C Tran 1 , Arya S Tamaskar 1 , Jing Zhao 1, 3 , Yutong Zhao 1, 3
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2021-01-05 , DOI: 10.1002/jcp.30253 Lian Li 1 , Jianxin Wei 2 , Tomeka L Suber 2 , Qinmao Ye 1 , Jiaxing Miao 1 , Shuang Li 2 , Sarah J Taleb 1 , Kevin C Tran 1 , Arya S Tamaskar 1 , Jing Zhao 1, 3 , Yutong Zhao 1, 3
Affiliation
|
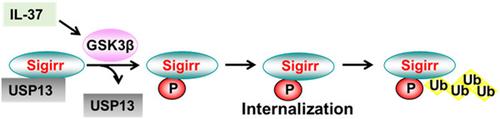
Interleukin (IL)‐37 diminishes a variety of inflammatory responses through ligation to its receptor IL‐1R8/Sigirr. Sigirr is a Toll like receptor/IL‐1R family member. We have shown that Sigirr is not stable in response to IL‐37 treatment. IL‐37‐induced Sigirr degradation is mediated by the ubiquitin‐proteasome system, and the process is reversed by a deubiquitinase, USP13. However, the molecular mechanisms by which USP13 regulates Sigirr stability have not been revealed. In this study, we investigate the roles of glycogen synthesis kinase 3β (GSK3β) in Sigirr phosphorylation and stability. IL‐37 stimulation induced Sigirr phosphorylation and degradation, as well as activation of GSK3β. Inhibition of GSK3β attenuated IL‐37‐induced Sigirr phosphorylation, while exogenous expressed GSK3β promoted Sigirr phosphorylation at threonine (T)372 residue. Sigirr association with GSK3β was detected. Amino acid residues 51–101 in GSK3β were identified as the Sigirr binding domain. These data indicate that GSK3β mediates IL‐37‐induced threonine phosphorylation of Sigirr. Further, we investigated the role of GSK3β‐mediated phosphorylation of Sigirr in Sigirr degradation. Inhibition of GSK3β attenuated IL‐37‐induced Sigirr degradation, while T372 mutant of Sigirr was resistant to IL‐37‐mediated degradation. Furthermore, inhibition of Sigirr phosphorylation prevented Sigirr internalization and association with USP13, suggesting GSK3β promotes Sigirr degradation through disrupting Sigirr association with USP13.
中文翻译:

IL-37 诱导的糖原合酶激酶 3β 激活促进肺上皮细胞的 IL-1R8/Sigirr 磷酸化、内化和降解
白细胞介素 (IL)-37 通过与其受体 IL-1R8/Sigirr 的连接来减少各种炎症反应。Sigirr 是 Toll 样受体/IL-1R 家族成员。我们已经证明 Sigirr 对 IL-37 治疗的反应不稳定。IL-37 诱导的 Sigirr 降解由泛素-蛋白酶体系统介导,而该过程被去泛素酶 USP13 逆转。然而,USP13 调节 Sigirr 稳定性的分子机制尚未揭示。在本研究中,我们研究了糖原合成激酶 3β (GSK3β) 在 Sigirr 磷酸化和稳定性中的作用。IL-37 刺激诱导 Sigirr 磷酸化和降解,以及 GSK3β 的激活。GSK3β的抑制减弱了IL-37诱导的Sigirr磷酸化,而外源表达的GSK3β促进了苏氨酸(T)372残基处的Sigirr磷酸化。检测到 Sigirr 与 GSK3β 的关联。GSK3β中的氨基酸残基51-101被鉴定为Sigirr结合域。这些数据表明 GSK3β 介导 IL-37 诱导的 Sigirr 苏氨酸磷酸化。此外,我们研究了 GSK3β 介导的 Sigirr 磷酸化在 Sigirr 降解中的作用。GSK3β的抑制减弱了IL-37诱导的Sigirr降解,而Sigirr的T372突变体对IL-37介导的降解具有抗性。此外,抑制 Sigirr 磷酸化阻止了 Sigirr 内化和与 USP13 的结合,表明 GSK3β 通过破坏 Sigirr 与 USP13 的结合来促进 Sigirr 降解。这些数据表明 GSK3β 介导 IL-37 诱导的 Sigirr 苏氨酸磷酸化。此外,我们研究了 GSK3β 介导的 Sigirr 磷酸化在 Sigirr 降解中的作用。GSK3β的抑制减弱了IL-37诱导的Sigirr降解,而Sigirr的T372突变体对IL-37介导的降解具有抗性。此外,抑制 Sigirr 磷酸化阻止了 Sigirr 内化和与 USP13 的结合,表明 GSK3β 通过破坏 Sigirr 与 USP13 的结合来促进 Sigirr 降解。这些数据表明 GSK3β 介导 IL-37 诱导的 Sigirr 苏氨酸磷酸化。此外,我们研究了 GSK3β 介导的 Sigirr 磷酸化在 Sigirr 降解中的作用。GSK3β的抑制减弱了IL-37诱导的Sigirr降解,而Sigirr的T372突变体对IL-37介导的降解具有抗性。此外,抑制 Sigirr 磷酸化阻止了 Sigirr 内化和与 USP13 的结合,表明 GSK3β 通过破坏 Sigirr 与 USP13 的结合来促进 Sigirr 降解。
更新日期:2021-01-05
中文翻译:

IL-37 诱导的糖原合酶激酶 3β 激活促进肺上皮细胞的 IL-1R8/Sigirr 磷酸化、内化和降解
白细胞介素 (IL)-37 通过与其受体 IL-1R8/Sigirr 的连接来减少各种炎症反应。Sigirr 是 Toll 样受体/IL-1R 家族成员。我们已经证明 Sigirr 对 IL-37 治疗的反应不稳定。IL-37 诱导的 Sigirr 降解由泛素-蛋白酶体系统介导,而该过程被去泛素酶 USP13 逆转。然而,USP13 调节 Sigirr 稳定性的分子机制尚未揭示。在本研究中,我们研究了糖原合成激酶 3β (GSK3β) 在 Sigirr 磷酸化和稳定性中的作用。IL-37 刺激诱导 Sigirr 磷酸化和降解,以及 GSK3β 的激活。GSK3β的抑制减弱了IL-37诱导的Sigirr磷酸化,而外源表达的GSK3β促进了苏氨酸(T)372残基处的Sigirr磷酸化。检测到 Sigirr 与 GSK3β 的关联。GSK3β中的氨基酸残基51-101被鉴定为Sigirr结合域。这些数据表明 GSK3β 介导 IL-37 诱导的 Sigirr 苏氨酸磷酸化。此外,我们研究了 GSK3β 介导的 Sigirr 磷酸化在 Sigirr 降解中的作用。GSK3β的抑制减弱了IL-37诱导的Sigirr降解,而Sigirr的T372突变体对IL-37介导的降解具有抗性。此外,抑制 Sigirr 磷酸化阻止了 Sigirr 内化和与 USP13 的结合,表明 GSK3β 通过破坏 Sigirr 与 USP13 的结合来促进 Sigirr 降解。这些数据表明 GSK3β 介导 IL-37 诱导的 Sigirr 苏氨酸磷酸化。此外,我们研究了 GSK3β 介导的 Sigirr 磷酸化在 Sigirr 降解中的作用。GSK3β的抑制减弱了IL-37诱导的Sigirr降解,而Sigirr的T372突变体对IL-37介导的降解具有抗性。此外,抑制 Sigirr 磷酸化阻止了 Sigirr 内化和与 USP13 的结合,表明 GSK3β 通过破坏 Sigirr 与 USP13 的结合来促进 Sigirr 降解。这些数据表明 GSK3β 介导 IL-37 诱导的 Sigirr 苏氨酸磷酸化。此外,我们研究了 GSK3β 介导的 Sigirr 磷酸化在 Sigirr 降解中的作用。GSK3β的抑制减弱了IL-37诱导的Sigirr降解,而Sigirr的T372突变体对IL-37介导的降解具有抗性。此外,抑制 Sigirr 磷酸化阻止了 Sigirr 内化和与 USP13 的结合,表明 GSK3β 通过破坏 Sigirr 与 USP13 的结合来促进 Sigirr 降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号