Corrosion Science ( IF 7.4 ) Pub Date : 2021-01-05 , DOI: 10.1016/j.corsci.2021.109236 B.S. Hou , Q.H. Zhang , Y.Y. Li , G.Y. Zhu , Y. Lei , X. Wang , H.F. Liu , G.A. Zhang
|
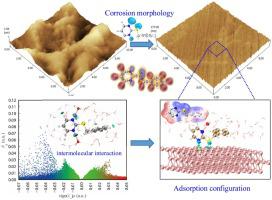
In this work, with 2-mercaptopyrimidine (MP) as the precursor, a pyrimidine derivative, 2-benzylthiopyrimidine (BTP), is developed as inhibitor to inhibit the corrosion of carbon steel in CO2-containing environment. Electrochemical measurements indicate that MP and BTP present high inhibition performance, especially for BTP with an inhibition efficiency of 99.82 %. Theoretical calculations show that the protonation of MP and BTP hardly occurs. The benzyl substituent in BTP enhances its adsorption ability by preventing tautomeric transformation and forming hydrophobic structure. Both MP and BTP molecules can adsorb on Fe surface in a nearly vertical orientation through the bonding of N and S atoms.
中文翻译:

基于实验和理论计算,深入探讨嘧啶衍生物对含CO 2环境中碳钢腐蚀的抑制机理
在这项工作中,以2-巯基嘧啶(MP)为前体,开发了嘧啶衍生物2-苄硫基嘧啶(BTP)作为抑制剂,以抑制碳钢在含CO 2的环境中的腐蚀。电化学测量表明,MP和BTP具有很高的抑制性能,特别是对于BTP而言,抑制效率为99.82%。理论计算表明,MP和BTP的质子化几乎不发生。BTP中的苄基取代基通过防止互变异构转变并形成疏水结构来增强其吸附能力。MP和BTP分子都可以通过N和S原子的键合以几乎垂直的方向吸附在Fe表面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号