当前位置:
X-MOL 学术
›
Comput. Struct. Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational design of noncanonical amino acid-based thioether staples at N/C-terminal domains of multi-modular pullulanase for thermostabilization in enzyme catalysis
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-01-05 , DOI: 10.1016/j.csbj.2020.12.043 Jiahua Bi 1 , Xiaoran Jing 1 , Lunjie Wu 1 , Xia Zhou 1 , Jie Gu 1 , Yao Nie 1, 2 , Yan Xu 1, 3
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-01-05 , DOI: 10.1016/j.csbj.2020.12.043 Jiahua Bi 1 , Xiaoran Jing 1 , Lunjie Wu 1 , Xia Zhou 1 , Jie Gu 1 , Yao Nie 1, 2 , Yan Xu 1, 3
Affiliation
|
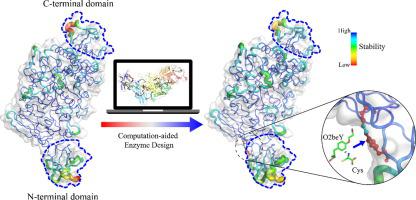
Enzyme thermostabilization is considered a critical and often obligatory step in biosynthesis, because thermostability is a significant property of enzymes that can be used to evaluate their feasibility for industrial applications. However, conventional strategies for thermostabilizing enzymes generally introduce non-covalent interactions and/or natural covalent bonds caused by natural amino acid substitutions, and the trade-off between the activity and stability of enzymes remains a challenge. Here, we developed a computationally guided strategy for constructing thioether staples by incorporating noncanonical amino acid (ncAA) into the more flexible N/C-terminal domains of the multi-modular pullulanase from (BtPul) to enhance its thermostability. First, potential thioether staples located in the N/C-terminal domains of BtPul were predicted using RosettaMatch. Next, eight variants involving stable thioether staples were precisely predicted using FoldX and Rosetta ddg_monomer. Six positive variants were obtained, of which T73(O2beY)-171C had a 157% longer half-life at 70 °C and an increase of 7.0 °C in , when compared with the wild-type (WT). T73(O2beY)-171C/T126F/A72R exhibited an even more improved thermostability, with a 211% increase in half-life at 70 °C and a 44% enhancement in enzyme activity compared with the WT, which was attributed to further optimization of the local interaction network. This work introduces and validates an efficient strategy for enhancing the thermostability and activity of multi-modular enzymes.
中文翻译:

多模块普鲁兰酶 N/C 末端结构域基于非规范氨基酸的硫醚主链的计算设计,用于酶催化中的热稳定性
酶的热稳定性被认为是生物合成中的关键且通常是必需的步骤,因为热稳定性是酶的一个重要特性,可用于评估其工业应用的可行性。然而,传统的热稳定酶策略通常会引入由天然氨基酸取代引起的非共价相互作用和/或天然共价键,酶的活性和稳定性之间的权衡仍然是一个挑战。在这里,我们开发了一种计算指导策略,通过将非规范氨基酸 (ncAA) 纳入多模块支链淀粉酶 (BtPul) 更灵活的 N/C 端结构域中,以增强其热稳定性,从而构建硫醚主链。首先,使用 RosettaMatch 预测位于 BtPul N/C 末端结构域的潜在硫醚主链。接下来,使用 FoldX 和 Rosetta ddg_monomer 精确预测了涉及稳定硫醚主链的八种变体。获得了 6 个阳性变体,其中 T73(O2beY)-171C 与野生型 (WT) 相比,70 °C 下的半衰期延长了 157%,并且增加了 7.0 °C。 T73(O2beY)-171C/T126F/A72R 表现出更高的热稳定性,与 WT 相比,70 °C 下的半衰期延长了 211%,酶活性提高了 44%,这归因于进一步优化本地交互网络。这项工作介绍并验证了一种增强多模块酶的热稳定性和活性的有效策略。
更新日期:2021-01-05
中文翻译:

多模块普鲁兰酶 N/C 末端结构域基于非规范氨基酸的硫醚主链的计算设计,用于酶催化中的热稳定性
酶的热稳定性被认为是生物合成中的关键且通常是必需的步骤,因为热稳定性是酶的一个重要特性,可用于评估其工业应用的可行性。然而,传统的热稳定酶策略通常会引入由天然氨基酸取代引起的非共价相互作用和/或天然共价键,酶的活性和稳定性之间的权衡仍然是一个挑战。在这里,我们开发了一种计算指导策略,通过将非规范氨基酸 (ncAA) 纳入多模块支链淀粉酶 (BtPul) 更灵活的 N/C 端结构域中,以增强其热稳定性,从而构建硫醚主链。首先,使用 RosettaMatch 预测位于 BtPul N/C 末端结构域的潜在硫醚主链。接下来,使用 FoldX 和 Rosetta ddg_monomer 精确预测了涉及稳定硫醚主链的八种变体。获得了 6 个阳性变体,其中 T73(O2beY)-171C 与野生型 (WT) 相比,70 °C 下的半衰期延长了 157%,并且增加了 7.0 °C。 T73(O2beY)-171C/T126F/A72R 表现出更高的热稳定性,与 WT 相比,70 °C 下的半衰期延长了 211%,酶活性提高了 44%,这归因于进一步优化本地交互网络。这项工作介绍并验证了一种增强多模块酶的热稳定性和活性的有效策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号