Catalysis Today ( IF 5.2 ) Pub Date : 2021-01-05 , DOI: 10.1016/j.cattod.2020.11.021 Fatima Jalid , Tuhin S. Khan , M. Ali Haider
|
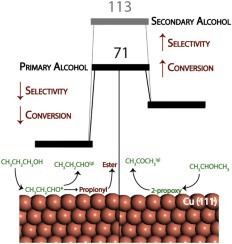
Density functional theory (DFT) simulations are conducted to understand the differentiation of activity and selectivity trends between primary and secondary alcohols towards the non-oxidative dehydrogenation (NODH) reaction. Propanol and isopropanol are employed as model molecules to explore the NODH on the (111) facets of Cu. The initial dehydrogenation of propanol via the hydroxyl route exhibits a higher barrier (96.8 kJ/mol) as compared to that of isopropanol (61.4 kJ/mol). Furthermore, the second dehydrogenation step resulting in the formation of the carbonyl compound is also calculated to show higher barrier of primary alcohol (83 kJ/mol) than secondary alcohol (51.3 kJ/mol). Overall, for the consecutive dehydrogenation steps, propanol is observed to show higher activation barriers as opposed to that of isopropanol, indicating towards a higher conversion rate for isopropanol, similar to the trend observed experimentally. However, the subsequent dehydrogenation of the carbonyl compound formed shows a barrier of 112.8 kJ/mol for acetone and that of 70.6 kJ/mol for acetaldehyde owing to the higher stability of ketone. The formation of ester from the propionyl produced upon acetaldehyde dehydrogenation is calculated to show the lowest barrier (64.6 kJ/mol) amongst the dehydrogenation steps of primary alcohol dehydrogenation route, suggesting towards lower selectivity in case of NODH of propanol.
中文翻译:

深入了解铜催化剂上伯醇和仲醇的非氧化脱氢活性和选择性趋势
进行密度泛函理论(DFT)模拟以了解伯醇和仲醇对非氧化脱氢(NODH)反应的活性和选择性趋势的差异。丙醇和异丙醇用作模型分子,以探索Cu(111)晶面上的NODH。与异丙醇(61.4 kJ / mol)相比,通过羟基途径进行的丙醇初始脱氢显示出更高的势垒(96.8 kJ / mol)。此外,还计算出导致羰基化合物形成的第二脱氢步骤显示出伯醇(83kJ / mol)比仲醇(51.3kJ / mol)更高的阻挡层。总体而言,对于连续的脱氢步骤,观察到丙醇与异丙醇相比显示出更高的活化能垒,表明异丙醇的转化率更高,类似于实验观察到的趋势。然而,由于酮的更高的稳定性,随后形成的羰基化合物的脱氢显示出对于丙酮的阻挡层为112.8kJ / mol,对于乙醛的阻挡层为70.6kJ / mol。计算出在乙醛脱氢路线的脱氢步骤中,由乙醛脱氢产生的丙酰基形成的酯显示出最低的势垒(64.6 kJ / mol),这表明在丙醇的NODH情况下选择性降低。由于酮的较高稳定性,乙醛为6 kJ / mol。计算出在乙醛脱氢路线的脱氢步骤中,由乙醛脱氢产生的丙酰基形成的酯显示出最低的势垒(64.6 kJ / mol),这表明在丙醇的NODH情况下选择性降低。由于酮的较高稳定性,乙醛为6 kJ / mol。计算出在乙醛脱氢路线的脱氢步骤中,由乙醛脱氢产生的丙酰基形成的酯显示出最低的势垒(64.6 kJ / mol),这表明在丙醇的NODH情况下选择性降低。









































 京公网安备 11010802027423号
京公网安备 11010802027423号