当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Concise Stereoselective Synthesis of β-Hydroxy-γ-lactones: (4R,5R)-4-Hydroxy-γ-decalactone from the Japanese Orange Fly and Enantiomers of Arachnid Harvestmen Isolates
Journal of Natural Products ( IF 5.1 ) Pub Date : 2021-01-04 , DOI: 10.1021/acs.jnatprod.0c01207 Ashvin J Gangani 1 , Praveen Kumar 1 , Rodney A Fernandes 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2021-01-04 , DOI: 10.1021/acs.jnatprod.0c01207 Ashvin J Gangani 1 , Praveen Kumar 1 , Rodney A Fernandes 1
Affiliation
|
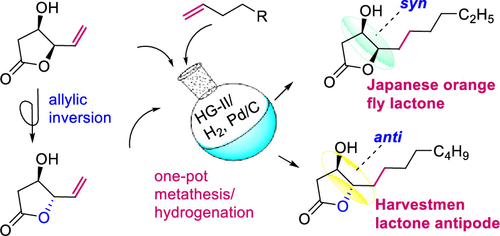
The naturally occurring (4R,5R)-4-hydroxy-γ-decalactone from the Japanese orange fly and the antipode of (4S,5R)-4-hydroxy-γ-dodecalactone from the harvestmen arachnid and their stereoisomers are synthesized from the chiral pool material d-glucono-δ-lactone in a few steps. The one-pot conversion of the latter to γ-vinyl-β-hydroxy-γ-lactone, cross-metathesis with requisite olefin, and hydrogenation enabled the synthesis of syn-lactones in just a two-pot operation. An additional efficient Pd-catalyzed allylic isomerization of γ-vinyl-β-hydroxy-γ-lactone led to the anti-lactones in high yields.
中文翻译:

β-羟基-γ-内酯的简明立体选择性合成:来自日本橙蝇的 (4R,5R)-4-羟基-γ-癸内酯和蛛形纲动物分离物的对映异构体
来自日本橙蝇的天然存在的 (4 R ,5 R )-4-羟基-γ-十内酯和来自收割机蛛形纲动物的 (4 S ,5 R )-4-羟基-γ-十二内酯及其立体异构体是由手性池材料d-葡萄糖酸-δ-内酯分几步合成。后者的一锅转化为γ-乙烯基-β羟基- γ内酯,交叉复分解以必要的烯烃和氢化启用的合成顺式内酯在短短的两锅操作。γ-乙烯基-β-羟基-γ-内酯的额外有效Pd催化烯丙基异构化导致高产率的抗-内酯。
更新日期:2021-01-22
中文翻译:

β-羟基-γ-内酯的简明立体选择性合成:来自日本橙蝇的 (4R,5R)-4-羟基-γ-癸内酯和蛛形纲动物分离物的对映异构体
来自日本橙蝇的天然存在的 (4 R ,5 R )-4-羟基-γ-十内酯和来自收割机蛛形纲动物的 (4 S ,5 R )-4-羟基-γ-十二内酯及其立体异构体是由手性池材料d-葡萄糖酸-δ-内酯分几步合成。后者的一锅转化为γ-乙烯基-β羟基- γ内酯,交叉复分解以必要的烯烃和氢化启用的合成顺式内酯在短短的两锅操作。γ-乙烯基-β-羟基-γ-内酯的额外有效Pd催化烯丙基异构化导致高产率的抗-内酯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号