当前位置:
X-MOL 学术
›
J. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of dihydroxy telechelic oligomers of β‐butyrolactone catalyzed by titanium(IV)‐alkoxides and their use as macrodiols in polyurethane chemistry
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2021-01-03 , DOI: 10.1002/pol.20200780 Hagen J. Altmann 1 , Martin R. Machat 2 , Aurel Wolf 2 , Christoph Gürtler 2 , Dongren Wang 1 , Michael R. Buchmeiser 1, 3
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2021-01-03 , DOI: 10.1002/pol.20200780 Hagen J. Altmann 1 , Martin R. Machat 2 , Aurel Wolf 2 , Christoph Gürtler 2 , Dongren Wang 1 , Michael R. Buchmeiser 1, 3
Affiliation
|
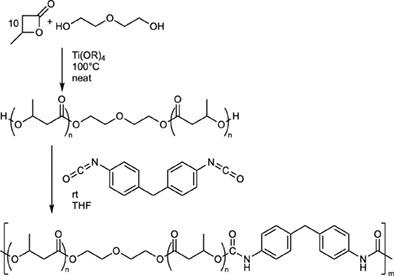
We report on a solvent‐free approach for the synthesis of low molecular weight, α,ω‐dihydroxy telechelic poly(β‐butyrolactone). In the presence of Ti(IV) alkoxides, mixtures of β‐butyrolactone and diols, like di‐ or triethylene glycol, were reacted in ratios between 4:1 and 10:1. The oligomerization proceeds at elevated temperatures (80–100°C). Different alkoxide substituents (R = Me, iPr, tBu) of the Ti(IV)(OR)4 catalyst were investigated. The resulting oligomers were characterized by nuclear magnetic resonance (NMR), infra‐red (IR), gel‐permeation chromatography (GPC), titration, and matrix‐assisted laser desorption‐time‐of‐flight mass spectrometry (MALDI‐ToF‐MS) analysis. Aside from low molecular weight products, special effort was devoted to achieve high O‐acyl cleavage selectivity and to circumvent the formation of unsaturated end‐groups in order to form exclusively dihydroxy‐telechelic oligomers. Optimized results in terms of selectivity and reaction rates were achieved at 100°C using catalyst loadings of 0.2 mol% with respect to the monomer. The molecular weights determined by GPC were in good accordance with the ratio of monomer to diol used, confirming successful oligomer formation. Polyurethanes prepared from crude macrodiols without any additional catalyst feature molecular weights up to 50,000 g/mol. The reported work serves as concept to utilize β‐lactones for tailored polyol synthesis; the resulting products are suitable for polyurethane chemistry.
中文翻译:

钛(IV)-醇盐催化的β-丁内酯二羟基远螯低聚物的合成及其在聚氨酯化学中作为大分子二醇的用途
我们报告了一种无溶剂合成低分子量α,ω-二羟基远螯聚(β-丁内酯)的方法。在Ti(IV)醇盐的存在下,β-丁内酯与二醇的混合物(例如二甘醇或三甘醇)以4:1至10:1的比例反应。低聚反应在高温(80–100°C)下进行。研究了Ti(IV)(OR)4催化剂的不同醇盐取代基(R = Me,i Pr,t Bu)。所形成的低聚物的特征在于核磁共振(NMR),红外(IR),凝胶渗透色谱(GPC),滴定和基质辅助激光解吸飞行时间质谱(MALDI-ToF-MS )分析。除了低分子量产品外,还致力于实现高分子量。O-酰基裂解选择性并绕开不饱和端基的形成,以便仅形成二羟基-端粒寡聚体。就选择性和反应速率而言,在相对于单体为0.2摩尔%的催化剂负载量下获得了优化的结果。通过GPC测定的分子量与所用单体与二醇的比例非常吻合,证实成功形成了低聚物。由粗大二醇制备的聚氨酯无需任何其他催化剂,其分子量最高可达50,000 g / mol。报道的工作是利用β-内酯进行量身定制的多元醇合成的概念。所得产物适用于聚氨酯化学。
更新日期:2021-02-01
中文翻译:

钛(IV)-醇盐催化的β-丁内酯二羟基远螯低聚物的合成及其在聚氨酯化学中作为大分子二醇的用途
我们报告了一种无溶剂合成低分子量α,ω-二羟基远螯聚(β-丁内酯)的方法。在Ti(IV)醇盐的存在下,β-丁内酯与二醇的混合物(例如二甘醇或三甘醇)以4:1至10:1的比例反应。低聚反应在高温(80–100°C)下进行。研究了Ti(IV)(OR)4催化剂的不同醇盐取代基(R = Me,i Pr,t Bu)。所形成的低聚物的特征在于核磁共振(NMR),红外(IR),凝胶渗透色谱(GPC),滴定和基质辅助激光解吸飞行时间质谱(MALDI-ToF-MS )分析。除了低分子量产品外,还致力于实现高分子量。O-酰基裂解选择性并绕开不饱和端基的形成,以便仅形成二羟基-端粒寡聚体。就选择性和反应速率而言,在相对于单体为0.2摩尔%的催化剂负载量下获得了优化的结果。通过GPC测定的分子量与所用单体与二醇的比例非常吻合,证实成功形成了低聚物。由粗大二醇制备的聚氨酯无需任何其他催化剂,其分子量最高可达50,000 g / mol。报道的工作是利用β-内酯进行量身定制的多元醇合成的概念。所得产物适用于聚氨酯化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号