当前位置:
X-MOL 学术
›
Can. J. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isothermal redox cycling of A‐ and B‐site substituted manganite‐based perovskites for CO2 conversion
The Canadian Journal of Chemical Engineering ( IF 1.6 ) Pub Date : 2021-01-02 , DOI: 10.1002/cjce.24019 Pradeep Shrestha 1 , Mahesh Muraleedharan Nair 1 , Nader Mahinpey 1
The Canadian Journal of Chemical Engineering ( IF 1.6 ) Pub Date : 2021-01-02 , DOI: 10.1002/cjce.24019 Pradeep Shrestha 1 , Mahesh Muraleedharan Nair 1 , Nader Mahinpey 1
Affiliation
|
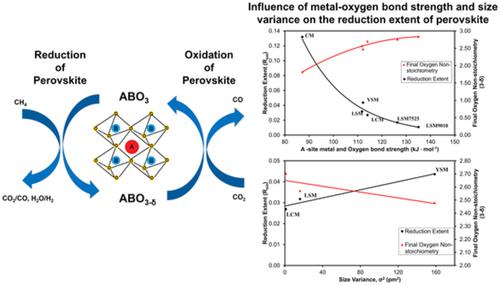
Conversion of CO2 into fuels and valuable chemicals can simultaneously address two major global issues: climate change and the increasing energy demand. Most of the previous studies regarding the thermochemical splitting of CO2 into CO have been carried out at elevated temperatures of up to 1400°C. In this study, under isothermal reaction conditions, manganite‐based perovskites are used to convert CO2 to CO in a thermogravimetric analyzer (TGA). In addition, the use of CH4 as a reducing agent has lowered the reaction temperatures (≤900°C). This study primarily focuses on the influence of A‐ and B‐site substitution on the redox performances of the manganite‐based perovskites. Based on the redox performances of four samples (CaMnO3, La0.5Ca0.5MnO3, La0.5Sr0.5MnO3, and Y0.5Sr0.5MnO3) with different A‐site compositions, La0.5Sr0.5MnO3 displays the best stability. Similarly, in the La1‐xSrxMnO3 perovskite family (x = 0.5, 0.25, and 0.10), the performance of La0.5Sr0.5MnO3 outshines the other two compositions. It is observed that as the Sr content increases, the activation energy for reduction decreases. Substituting Mn with Al and Fe in the B‐site of the La1‐xSrxMnO3 perovskite, did not enhance the performance. The perovskite materials investigated have shown decrease in activity after the first cycle, possibly due to sintering and powder densification. However, after the first cycle, the perovskites illustrated good stability for 10 redox cycles. In this paper, size variance and metal‐oxygen bond strength provide the best explanation for the trends observed during the reduction of the perovskites.
中文翻译:

A和B位置取代锰铁矿的钙钛矿的等温氧化还原循环用于CO2转化
将CO 2转化为燃料和有价值的化学物质可以同时解决两个主要的全球性问题:气候变化和不断增长的能源需求。先前有关将CO 2热化学分解为CO的大多数研究都是在高达1400°C的高温下进行的。在这项研究中,在等温反应条件下,基于锰铁矿的钙钛矿被用于在热重分析仪(TGA)中将CO 2转化为CO。另外,使用CH 4作为还原剂降低了反应温度(≤900℃)。这项研究主要集中在A位和B位取代对锰基钙钛矿的氧化还原性能的影响上。基于四个样品(CaMnO的氧化还原性能3,具有不同A部位组成的La 0.5 Ca 0.5 MnO 3,La 0.5 Sr 0.5 MnO 3和Y 0.5 Sr 0.5 MnO 3),La 0.5 Sr 0.5 MnO 3表现出最佳的稳定性。同样,在La 1-x Sr x MnO 3钙钛矿家族(x = 0.5、0.25和0.10)中,La 0.5 Sr 0.5 MnO 3的性能胜过其他两个组成部分。观察到随着Sr含量的增加,用于还原的活化能降低。在La 1 x Sr x MnO 3钙钛矿的B位中用Al和Fe代替Mn并不能提高性能。所研究的钙钛矿材料在第一个循环后显示出活性降低,可能是由于烧结和粉末致密化所致。然而,在第一个循环之后,钙钛矿显示出在10个氧化还原循环中的良好稳定性。在本文中,尺寸变化和金属-氧键强度为钙钛矿还原过程中观察到的趋势提供了最好的解释。
更新日期:2021-01-02
中文翻译:

A和B位置取代锰铁矿的钙钛矿的等温氧化还原循环用于CO2转化
将CO 2转化为燃料和有价值的化学物质可以同时解决两个主要的全球性问题:气候变化和不断增长的能源需求。先前有关将CO 2热化学分解为CO的大多数研究都是在高达1400°C的高温下进行的。在这项研究中,在等温反应条件下,基于锰铁矿的钙钛矿被用于在热重分析仪(TGA)中将CO 2转化为CO。另外,使用CH 4作为还原剂降低了反应温度(≤900℃)。这项研究主要集中在A位和B位取代对锰基钙钛矿的氧化还原性能的影响上。基于四个样品(CaMnO的氧化还原性能3,具有不同A部位组成的La 0.5 Ca 0.5 MnO 3,La 0.5 Sr 0.5 MnO 3和Y 0.5 Sr 0.5 MnO 3),La 0.5 Sr 0.5 MnO 3表现出最佳的稳定性。同样,在La 1-x Sr x MnO 3钙钛矿家族(x = 0.5、0.25和0.10)中,La 0.5 Sr 0.5 MnO 3的性能胜过其他两个组成部分。观察到随着Sr含量的增加,用于还原的活化能降低。在La 1 x Sr x MnO 3钙钛矿的B位中用Al和Fe代替Mn并不能提高性能。所研究的钙钛矿材料在第一个循环后显示出活性降低,可能是由于烧结和粉末致密化所致。然而,在第一个循环之后,钙钛矿显示出在10个氧化还原循环中的良好稳定性。在本文中,尺寸变化和金属-氧键强度为钙钛矿还原过程中观察到的趋势提供了最好的解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号