Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-01-02 , DOI: 10.1016/j.bmc.2020.115991 Diego A S Yamazaki 1 , Andrew M F Rozada 1 , Paula Baréa 1 , Elaine C Reis 1 , Ernani A Basso 1 , Maria Helena Sarragiotto 1 , Flávio A V Seixas 2 , Gisele F Gauze 1
|
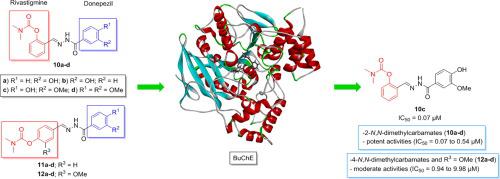
A novel series of arylcarbamate-N-acylhydrazones derivatives have been designed and synthesized as potential anti-cholinesterase agents. In vitro studies revealed that these compounds demonstrated selective for butyrylcholinesterase (BuChE) with potent inhibitory activity. The compounds 10a-d, 12b and 12d were the most potent BuChE inhibitors with IC50 values of 0.07–2.07 µM, highlighting the compound 10c (IC50 = 0.07 µM) which showed inhibitory activity 50 times greater than the reference drug donepezil (IC50 = 3.54 µM). The activity data indicates that the position of the carbamate group in the aromatic ring has a greater influence on the inhibitory activity of the derivatives. The enzyme kinetics studies indicate that the compound 10c has a non-competitive inhibition against BuChE with Ki value of 0.097 mM. Molecular modeling studies corroborated the in vitro inhibitory mode of interaction and show that compound 10c is stabilized into hBuChE by strong hydrogen bond interaction with Tyr128, π-π stacking interaction with Trp82 and CH⋯O interactions with His438, Gly121 and Glu197. Based on these data, compound 10c was identified as low-cost promising candidate for a drug prototype for AD treatment.
中文翻译:

作为有前途的 BuChE 抑制剂的新型芳基氨基甲酸酯-N-酰基腙衍生物:设计、合成、分子建模和生物学评价
已经设计并合成了一系列新的芳基氨基甲酸酯-N-酰基腙衍生物作为潜在的抗胆碱酯酶剂。体外研究表明,这些化合物对丁酰胆碱酯酶 (BuChE) 具有选择性,具有强效抑制活性。化合物10a-d、12b和12d是最有效的 BuChE 抑制剂,IC 50值为 0.07–2.07 µM,突出显示化合物10c (IC 50 = 0.07 µM) 的抑制活性是参考药物多奈哌齐 (IC 50 = 3.54 µM)。活性数据表明,芳香环中氨基甲酸酯基团的位置对衍生物的抑制活性影响较大。酶动力学研究表明,化合物10c对 BuChE 具有非竞争性抑制作用,Ki 值为 0.097 mM。分子模型研究证实了体外抑制相互作用模式,并表明化合物10c通过与 Tyr128 的强氢键相互作用、与 Trp82 的 π-π 堆积相互作用以及与 His438、Gly121 和 Glu197 的 CH⋯O 相互作用稳定在 hBuChE 中。基于这些数据,化合物 10c 被确定为 AD 治疗药物原型的低成本、有前景的候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号