Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2021-01-02 , DOI: 10.1016/j.jphotochem.2020.113122 Shin-ichi Nagaoka , Yuki Bandoh , Satoki Matsuhiroya , Kazumasa Inoue , Umpei Nagashima , Keishi Ohara
|
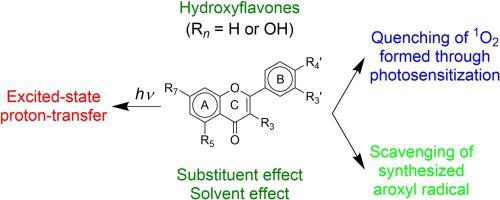
Substituent and solvent effects on activities of singlet-oxygen (1O2) quenching, free-radical scavenging and excited-state proton-transfer (ESPT) in hydroxyflavones were investigated by means of laser, stopped-flow and steady-state spectroscopies together with density functional calculations. Some correlations were found among the three reaction activities. Substitution of OH group at the 5-position in hydroxyflavones increases the ionization energy, hinders the electron transfer from the molecule to 1O2 and free-radicals, and causes deactivation of the 1O2 quenching and free-radical scavenging in ethanol, although the ESPT is very efficient. OH substitution at the 7-position has a negligible influence on the 1O2 quenching and free-radical scavenging activities, but induces solvent-assisted ESPT, which is deactivated by substitution of a catechol structure at B-ring. A synergetic effect between the electron transfer and ESPT-induced potential-surface distortion is likely present on the 1O2 quenching and free-radical scavenging activities. The 1O2 quenching activity of 3,3’,4’-trihydroxyflavone monotonously increases with increasing water concentration in the ethanol solution, whereas addition of a small amount of water respectively activates and deactivates the ESPT and free-radical scavenging, whose activity changes are saturated by addition of a large amount. The reason for these results can be explained in terms of the nodal-plane model, ionization energy difference and potential-curve distortion/displacement with regard to the ESPT and solvation.
中文翻译:

羟基黄酮中单线态氧猝灭,自由基清除和激发态质子转移之间的活性相关性:取代基和溶剂效应
研究了取代基和溶剂对羟基黄酮中单线态氧(1 O 2)猝灭,自由基清除和激发态质子转移(ESPT)活性的影响,采用激光,停流和稳态分光光度法以及密度泛函计算。在这三个反应活动之间发现了一些相关性。羟基黄酮中5位的OH基团取代会增加电离能,阻碍电子从分子转移到1 O 2和自由基,并导致1 O 2失活尽管ESPT非常有效,但可以在乙醇中进行淬灭和自由基清除。在7位上的OH取代对1 O 2猝灭和自由基清除活性的影响可忽略不计,但诱导了溶剂辅助的ESPT,该溶剂通过在B环上邻苯二酚结构的取代而失活。在1 O 2猝灭和自由基清除活性上可能存在电子转移与ESPT诱导的电位表面畸变之间的协同作用。的1 Ò 23,3',4'-三羟基黄酮的淬灭活性随乙醇溶液中水浓度的增加而单调增加,而加入少量水则分别激活和使ESPT和自由基清除,而其清除后的活性变化达到饱和大量。这些结果的原因可以用节面模型,电离能差和关于ESPT和溶剂化的电势曲线失真/位移来解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号