Journal of Molecular Liquids ( IF 6 ) Pub Date : 2021-01-02 , DOI: 10.1016/j.molliq.2020.115267 Ming Lin , Xianwei Hu , Jiangyu Yu , Youjian Yang , Aimin Liu , Zhongning Shi , Zhaowen Wang
|
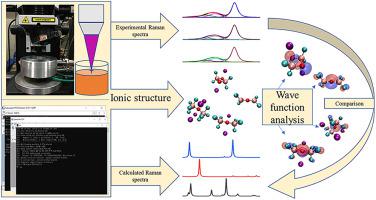
The ionic structure of the KF-AlF3-Al2O3 system was studied using in situ high-temperature Raman spectroscopy and quantum mechanics calculations. The complexes in the KF-AlF3-Al2O3 system with different molar ratios of KF to AlF3 were AlF63−, AlF52−, AlF4−, Al2OF4, Al2OF84−, Al2OF62−, and Al2O2F42−. The structures of Al2OF4, Al2OF84−, Al2OF62−, and Al2O2F42− were optimized considering that the counterion K+, Al2OF4 exhibits D2d point group symmetry, K4Al2OF8 and K2Al2OF6 exhibit C2v point group symmetry, and K2Al2O2F4 exhibits C2h point group symmetry. The experimental main Raman bands of Al2OF84−, Al2OF62−, and Al2O2F42− were at 495 cm−1, 465 cm−1, and 410 cm−1, respectively, after comparing their theoretical Raman spectra. The forms and contents of the oxygen-containing complex ions in the system were related to the molar ratio of KF to AlF3 and to the concentration of alumina. AlF63−, AlF52−, AlF4−, and Al2OF4 were found in the molten KF-AlF3-Al2O3 system with a KF-to-AlF3 molar ratio of 1.22 at a temperature of 1003 K. In the KF-AlF3-Al2O3 system with a KF-to-AlF3 molar ratio of 2 at a temperature of 1273 K, when the alumina concentration was less than or equal to 10 wt%, it contained AlF63−, AlF52−, AlF4−, Al2OF4, and Al2OF84−, and when the alumina concentration reached 12 wt%, Al2OF62− and Al2O2F42− appeared. In the KF-AlF3-Al2O3 system with a KF-to-AlF3 molar ratio of 3 at a temperature of 1273 K, when the alumina concentration was less than or equal to 10 wt%, the system contained AlF63−, AlF52−, AlF4−, Al2OF4, and Al2OF84−. When the alumina concentration increased to 14 wt%, Al2OF62− and Al2O2F42− began to appear but AlF4− disappeared. As the concentration of alumina increased, the contents of Al2OF62− and Al2O2F42− increased. The ionic reactions that formed the various oxygen-containing complexes were obtained. The occurrence of these chemical reactions was related to the molar ratio of KF to AlF3 in the system and the alumina concentration. When the KF-AlF3-Al2O3 molten system with KF-to-AlF3 molar ratios of 1.22, 2 and 3 were used as the aluminum electrolytes, the ion discharged at the anode was Al2OF84−(except for the system with a KF-to-AlF3 molar ratio of 1.22 in which the Al2OF4 was discharged at the anode), and the ion discharged at the cathode was Al2OF4 according to wave function analyses.
中文翻译:

KF-AlF 3 -Al 2 O 3体系中配合物的拉曼光谱和量子理论计算
利用原位高温拉曼光谱和量子力学计算研究了KF-AlF 3 -Al 2 O 3体系的离子结构。在KF-的AlF络合物3 -Al 2 ö 3与KF的到的AlF不同摩尔比系统3分别的AlF 6 3-,的AlF 5 2-,的AlF 4 -,AL 2 OF 4,AL 2 OF 8 4-, Al 2 OF 6 2−和Al 2 O 2 F 42−。考虑到抗衡离子K +,Al 2 OF 4表现出D 2d点群对称性,因此优化了Al 2 OF 4,Al 2 OF 8 4-,Al 2 OF 6 2-和Al 2 O 2 F 4 2-的结构。,K 4 Al 2 OF 8和K 2 Al 2 OF 6表现出C 2v点群对称性,而K 2 Al 2 O2 F 4具有C 2h点群对称性。Al的实验主要拉曼频带2的8 4-,AL 2 OF 6 2-,和Al 2 ö 2 ˚F 4 2-均在495厘米-1,465厘米-1,和410厘米-1分别,后比较他们的理论拉曼光谱。系统中含氧络合物离子的形式和含量与KF与AlF 3的摩尔比以及氧化铝的浓度有关。AlF 6 3−,AlF 5 2−,的AlF 4 - ,和Al 2 OF 4在熔融KF-的AlF发现3 -Al 2 ö 3与KF到的AlF系统3的1.22摩尔比的KF-的AlF的1003 K的温度下3在1273 K的温度下,KF与AlF 3的摩尔比为2的-Al 2 O 3系统,当氧化铝浓度小于或等于10 wt%时,包含AlF 6 3-和AlF 5 2-,的AlF 4 -,AL 2 OF 4和Al 2 OF在图8 4-中,当氧化铝浓度达到12重量%时,出现Al 2 OF 6 2-和Al 2 O 2 F 4 2-。在1273 K的温度下KF与AlF 3摩尔比为3的KF-AlF 3 -Al 2 O 3系统中,当氧化铝浓度小于或等于10 wt%时,系统包含AlF 6 3-,的AlF 5 2-,的AlF 4 -,AL 2 OF 4和Al 2 OF 8 4-。当氧化铝的浓度增加至14质量%,Al 2 OF 6 2-和Al 2 ö 2 ˚F 4 2-开始出现,但的AlF 4 -消失了。随着氧化铝浓度的增加,Al 2 OF 6 2-和Al 2 O 2 F 4 2-的含量增加。获得了形成各种含氧络合物的离子反应。这些化学反应的发生与体系中KF与AlF 3的摩尔比和氧化铝浓度有关。当KF-AlF 3使用KF与AlF 3摩尔比为1.22、2和3的-Al 2 O 3熔融体系作为铝电解质,在阳极处释放的离子为Al 2 OF 8 4-(具有KF的体系除外) -to-的AlF 3的1.22,其中Al的摩尔比2的4在阳极排出),并且离子在阴极为Al排出2 OF 4根据波函数的分析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号