Catalysis Today ( IF 5.2 ) Pub Date : 2021-01-02 , DOI: 10.1016/j.cattod.2020.12.012 Chinmaya Mirle , Veerababu Medabalmi , Kothandaraman Ramanujam
|
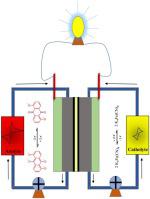
Among the aqueous organic redox flow batteries, quinone derivatives based redox molecules are commonly explored, due to their facile electron transfer ability. In this work, we have screened three redox active quinones (anthrarufin (AN), naphthazarin (NN), and juglone (JN)) for anolyte and cells were fabricated against K4Fe(CN)6 catholyte. The anolyte with more number of aromatic rings, i.e., AN is capable of better delocalizing the electrons and show more negative redox potential, thereby enhancing the operating voltage of the battery. AN exhibited a single step two-electron reduction process and delivered a capacity of 1.32 Ah L−1 at 20 mA cm-2 current density.
中文翻译:

用于含水碱性有机氧化还原液流电池的无交叉羟基取代的醌阳极电解液和亚铁氰化钾阴极电解液
在水性有机氧化还原液流电池中,基于醌衍生物的氧化还原分子由于其容易的电子传递能力而被普遍研究。在这项工作中,我们筛选了三种氧化还原活性醌(蒽醌(AN),萘达那林(NN)和ju(JN))作为阳极电解液,并针对K 4 Fe(CN)6阴极电解液制备了细胞。具有更多数目的芳环的阳极电解液,即AN能够更好地使电子离域并显示更多的负氧化还原电势,从而提高电池的工作电压。AN展示了单步两电子还原过程,并在20 mA cm -2的电流密度下提供了1.32 Ah L -1的容量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号