当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of the Artificial Nanostructure on the LiF Formation at the Solid–Electrolyte Interphase of Carbon-Based Anodes
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-12-31 , DOI: 10.1021/acsaem.0c02798 Katrine L. Svane 1 , Sebastian Zimmer Lefmann 1 , Mads Schousboe Vilmann 1 , Jan Rossmeisl 2 , Ivano E. Castelli 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-12-31 , DOI: 10.1021/acsaem.0c02798 Katrine L. Svane 1 , Sebastian Zimmer Lefmann 1 , Mads Schousboe Vilmann 1 , Jan Rossmeisl 2 , Ivano E. Castelli 1
Affiliation
|
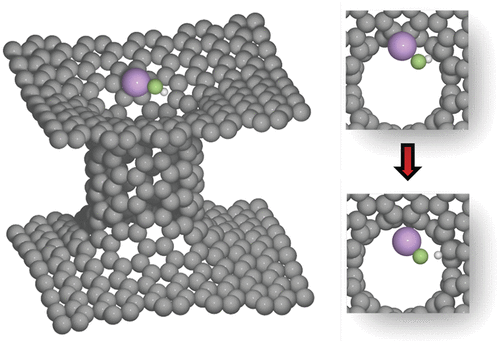
The solid–electrolyte interphase (SEI) is of crucial importance for the performance of Li-ion batteries. Here, density functional theory (DFT) calculations are used to study the formation of one of the simplest and early appearing components of the SEI layer, namely, LiF, which is produced by splitting HF impurities. The process is investigated on different models representing the basal and edge-planes of a graphitic anode, and on covalently connected carbon nanotubes and graphene sheets, known as pillared graphene. The results show that 2 Li atoms are required to bind F in the final state in order to make the reaction energetically favorable, or alternatively, a H atom must be preadsorbed. The Li adsorption energy, and thereby the Li coverage at a given potential, varies for the different carbon structures, demonstrating that the artificial nanostructure of the carbon can influence the formation of the SEI.
中文翻译:

人工纳米结构对碳基阳极固-电解质界面LiF形成的影响
固体电解质中间相(SEI)对于锂离子电池的性能至关重要。在这里,使用密度泛函理论(DFT)计算来研究SEI层中最简单且较早出现的成分之一LiF的形成,这是通过分解HF杂质而产生的。在代表石墨阳极基础平面和边缘平面的不同模型以及共价连接的碳纳米管和石墨烯片(称为柱状石墨烯)上研究了该过程。结果表明,为了使反应在能量上有利,需要2个Li原子以最终状态结合F,或者必须预先吸附H原子。Li的吸附能以及给定电势下的Li覆盖率随碳结构的不同而变化,
更新日期:2021-01-25
中文翻译:

人工纳米结构对碳基阳极固-电解质界面LiF形成的影响
固体电解质中间相(SEI)对于锂离子电池的性能至关重要。在这里,使用密度泛函理论(DFT)计算来研究SEI层中最简单且较早出现的成分之一LiF的形成,这是通过分解HF杂质而产生的。在代表石墨阳极基础平面和边缘平面的不同模型以及共价连接的碳纳米管和石墨烯片(称为柱状石墨烯)上研究了该过程。结果表明,为了使反应在能量上有利,需要2个Li原子以最终状态结合F,或者必须预先吸附H原子。Li的吸附能以及给定电势下的Li覆盖率随碳结构的不同而变化,









































 京公网安备 11010802027423号
京公网安备 11010802027423号