Cellular Signalling ( IF 4.4 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.cellsig.2020.109912 Andromachi Lambrianidou 1 , Evangelia Sereti 2 , Katerina Soupsana 3 , Chrysoula Komini 1 , Konstantinos Dimas 2 , Theoni Trangas 1
|
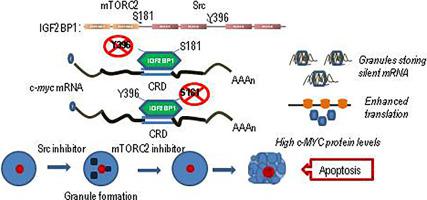
mTORC2 promotes cell survival by phosphorylating AKT and enhancing its activity. Inactivation of mTORC2 reduces viability through down-regulation of E2F1 caused by up-regulation of c-MYC. An additional target of mTORC2 is IGF2BP1, an oncofetal RNA binding protein expressed de novo in a wide array of malignancies. IGF2BP1 enhances c-MYC expression by protecting the coding region instability sequence (CRD) of its mRNA from endonucleolytic cleavage. Here we show that repression of mTORC2 signalling and prevention of Ser181 phosphorylation of IGF2BP1 enhanced translation and destabilization of the endogenous c-myc mRNA as well as the mRNA of reporter transcripts carrying the CRD sequence in frame. The consequent increase in c-MYC protein was accompanied by the emergence of an apoptotic c-MYC overexpressing population. On the other hand, preventing phosphorylation of IGF2BP1 on Tyr396 by Src kinase caused the accumulation of translationally silent transcripts through sequestration by IGF2BP1 into cytoplasmic granules. The apoptotic effect of mTORC2 signalling deprivation was augmented when preceded by inhibition of IGF2BP1 phosphorylation by the Src kinase in concert with further increase of c-MYC levels because of enhanced translation of the previously stored mRNA only in the presence of IGF2BP1. Furthermore, the combined administration of mTORC2 and Src inhibitors exhibited synergism in delaying xenograft growth in female NOD.CB17-Prkdcscid/J mice. The above in vitro and in vivo findings may be applied for the induction of targeted apoptosis of cells expressing de novo the oncofetal protein IGF2BP1, a feature of aggressive malignancies resulting in a more focused anticancer therapeutic approach.
中文翻译:

mTORC2 部署 mRNA 结合蛋白 IGF2BP1 来调节 c-MYC 表达并促进细胞存活
mTORC2 通过磷酸化 AKT 并增强其活性来促进细胞存活。mTORC2 的失活通过由 c-MYC 上调引起的 E2F1 下调来降低活力。mTORC2 的另一个靶点是 IGF2BP1,这是一种在多种恶性肿瘤中从头表达的癌胎儿 RNA 结合蛋白。IGF2BP1通过保护其 mRNA 的编码区不稳定性序列 (CRD) 免受核酸内切酶切割而增强 c- MYC表达。在这里,我们表明抑制 mTORC2 信号传导和阻止 IGF2BP1 的 Ser181 磷酸化增强了内源性 c -myc的翻译和不稳定mRNA 以及在框架中携带 CRD 序列的报告转录物的 mRNA。随之而来的 c-MYC 蛋白增加伴随着凋亡 c-MYC 过表达群体的出现。另一方面,通过 Src 激酶阻止 Tyr396 上的 IGF2BP1 磷酸化导致通过 IGF2BP1 隔离到细胞质颗粒中积累翻译沉默的转录物。当 Src 激酶抑制 IGF2BP1 磷酸化之前,mTORC2 信号剥夺的凋亡效应与 c-MYC 水平的进一步增加相一致,因为仅在 IGF2BP1 存在下增强了先前储存的 mRNA 的翻译。此外,mTORC2 和 Src 抑制剂的联合给药在延缓女性 NOD.CB17-Prkdc 异种移植物生长方面表现出协同作用scid /J 小鼠。上述体外和体内研究结果可用于诱导从头表达癌胎蛋白 IGF2BP1 的细胞的靶向凋亡,这是侵袭性恶性肿瘤的一个特征,导致更集中的抗癌治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号