当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Web-Based Toolbox to Support Quantitative In-Vitro-to-In-Vivo Extrapolations (QIVIVE) within Nonanimal Testing Strategies
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-12-31 , DOI: 10.1021/acs.chemrestox.0c00307 Ans Punt 1 , Nicole Pinckaers 1 , Ad Peijnenburg 1 , Jochem Louisse 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-12-31 , DOI: 10.1021/acs.chemrestox.0c00307 Ans Punt 1 , Nicole Pinckaers 1 , Ad Peijnenburg 1 , Jochem Louisse 1
Affiliation
|
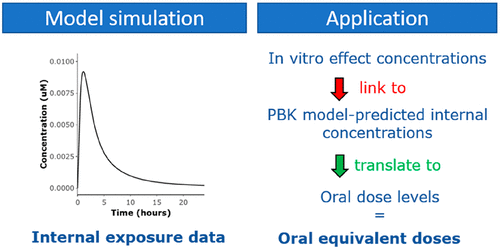
The goal of the present study was to develop an online web-based toolbox that contains generic physiologically based kinetic (PBK) models for rats and humans, including underlying calculation tools to predict plasma protein binding and tissue:plasma distribution, to be used for quantitative in-vitro-to-in-vivo extrapolations (QIVIVE). The PBK models within the toolbox allow first estimations of internal plasma and tissue concentrations of chemicals to be made, based on the logP and pKa of the chemicals and values for intestinal uptake and intrinsic hepatic clearance. As a case study, the toolbox was used to predict oral equivalent doses of in vitro ToxCast bioactivity data for the food additives methylparaben, propyl gallate, octyl gallate, and dodecyl gallate. These oral equivalent doses were subsequently compared with human exposure estimates, as a low tier assessment allowing prioritization for further assessment. The results revealed that daily intake levels of especially propyl gallate can lead to internal plasma concentrations that are close to in vitro biological effect concentrations, particularly with respect to the inhibition of human thyroid peroxidase (TPO). Estrogenic effects were not considered likely to be induced by the food additives, as daily exposure levels of the different compounds remained 2 orders of magnitude below the oral equivalent doses for in vitro estrogen receptor activation. Overall, the results of the study show how the toolbox, which is freely accessible through www.qivivetools.wur.nl, can be used to obtain initial internal dose estimates of chemicals and to prioritize chemicals for further assessment, based on the comparison of oral equivalent doses of in vitro biological activity data with human exposure levels.
中文翻译:

开发基于网络的工具箱以支持非动物测试策略中的定量体外到体内外推(QIVIVE)
本研究的目标是开发一个基于网络的在线工具箱,其中包含大鼠和人类的通用生理学动力学 (PBK) 模型,包括预测血浆蛋白结合和组织:血浆分布的基础计算工具,用于定量体外到体内外推(QIVIVE)。工具箱中的 PBK 模型可以根据化学物质的 logP 和 pKa 以及肠道摄取和内在肝脏清除率的值,首先估计化学物质的内部血浆和组织浓度。作为案例研究,该工具箱用于预测食品添加剂对羟基苯甲酸甲酯、没食子酸丙酯、没食子酸辛酯和没食子酸十二酯的体外 ToxCast 生物活性数据的口服等效剂量。随后将这些口服当量剂量与人类暴露估计值进行比较,作为低级评估,允许优先考虑进一步评估。结果表明,每日摄入没食子酸丙酯水平可导致体内血浆浓度接近体外生物效应浓度,特别是在抑制人甲状腺过氧化物酶(TPO)方面。雌激素效应不被认为是由食品添加剂引起的,因为不同化合物的每日暴露水平仍比体外雌激素受体激活的口服等效剂量低2个数量级。总体而言,研究结果表明,该工具箱(可通过 www.qivivetools.wur.nl 免费访问)可用于获取化学品的初始内部剂量估计值,并根据口服剂量的比较,确定化学品的优先顺序以进行进一步评估。体外生物活性数据与人体暴露水平的等效剂量。
更新日期:2021-02-15
中文翻译:

开发基于网络的工具箱以支持非动物测试策略中的定量体外到体内外推(QIVIVE)
本研究的目标是开发一个基于网络的在线工具箱,其中包含大鼠和人类的通用生理学动力学 (PBK) 模型,包括预测血浆蛋白结合和组织:血浆分布的基础计算工具,用于定量体外到体内外推(QIVIVE)。工具箱中的 PBK 模型可以根据化学物质的 logP 和 pKa 以及肠道摄取和内在肝脏清除率的值,首先估计化学物质的内部血浆和组织浓度。作为案例研究,该工具箱用于预测食品添加剂对羟基苯甲酸甲酯、没食子酸丙酯、没食子酸辛酯和没食子酸十二酯的体外 ToxCast 生物活性数据的口服等效剂量。随后将这些口服当量剂量与人类暴露估计值进行比较,作为低级评估,允许优先考虑进一步评估。结果表明,每日摄入没食子酸丙酯水平可导致体内血浆浓度接近体外生物效应浓度,特别是在抑制人甲状腺过氧化物酶(TPO)方面。雌激素效应不被认为是由食品添加剂引起的,因为不同化合物的每日暴露水平仍比体外雌激素受体激活的口服等效剂量低2个数量级。总体而言,研究结果表明,该工具箱(可通过 www.qivivetools.wur.nl 免费访问)可用于获取化学品的初始内部剂量估计值,并根据口服剂量的比较,确定化学品的优先顺序以进行进一步评估。体外生物活性数据与人体暴露水平的等效剂量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号