当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Surface Modified Live Biotherapeutic Products for Oral Delivery
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-12-30 , DOI: 10.1021/acsbiomaterials.0c01405 Ava M Vargason 1 , Aaron C Anselmo 1
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-12-30 , DOI: 10.1021/acsbiomaterials.0c01405 Ava M Vargason 1 , Aaron C Anselmo 1
Affiliation
|
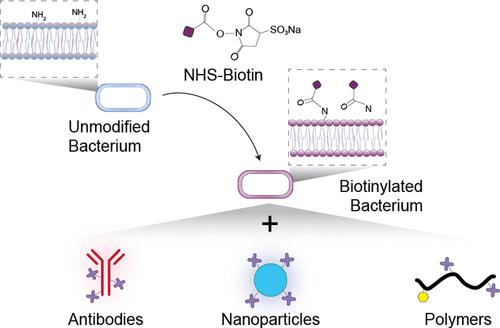
Live biotherapeutic products (LBPs), including symbiotic and genetically engineered bacteria, are a promising class of emerging therapeutics that are widely investigated both preclinically and clinically for their oral delivery to the gastrointestinal (GI) tract. One emergent delivery strategy involves the direct functionalization of LBP surfaces through noncovalent or covalent modifications to control LBP interactions with the GI microenvironment, thereby improving their viability, attachment, or therapeutic effect. However, unlike other therapeutic modalities, LBPs are living organisms which present two unique challenges for surface modifications: (1) this approach can directly interfere with key LBP biological processes (e.g., colonization, metabolite secretion) and (2) modification can be variable due to the dynamic nature of LBP surfaces. Collectively, these factors remain uncharacterized as they relate to the oral delivery of LBPs. Herein, we leverage our previously reported surface modification platform, which enables LBP surface-presentation of targeting ligands, to broadly evaluate and characterize surface modifications on LBPs. Specifically, we evaluate how LBP growth affects the dilution of surface-presented targeting ligands and the subsequent loss of specific target attachment over time. Next, we describe key surface modification parameters (e.g., concentration, residence time) that can be optimized to facilitate LBP target attachment. We then characterize how bioconjugation influences the suitability of LBPs for oral delivery by evaluating their growth, viability, storage, toxicity against mammalian cells, and in vivo colonization. Broadly, we describe key parameters that influence the performance of surface modified LBPs and subsequently outline an experimental pipeline for characterizing and evaluating their suitability for oral delivery.
中文翻译:

用于口服给药的表面改性活生物治疗产品的评价
活生物治疗产品(LBP),包括共生细菌和基因工程细菌,是一类很有前途的新兴疗法,在临床前和临床上对其口服递送至胃肠道(GI)进行了广泛研究。一种新兴的递送策略涉及通过非共价或共价修饰直接功能化 LBP 表面,以控制 LBP 与胃肠道微环境的相互作用,从而提高其活力、附着或治疗效果。然而,与其他治疗方式不同,LBP 是活的有机体,对表面修饰提出了两个独特的挑战:(1)这种方法可以直接干扰关键的 LBP 生物过程(例如,定植、代谢物分泌)和(2)修饰可能因表面修饰而变化。 LBP 表面的动态性质。总的来说,这些因素仍然没有被表征,因为它们与 LBP 的口服给药有关。在此,我们利用之前报道的表面修饰平台来广泛评估和表征 LBP 的表面修饰,该平台能够实现靶向配体的 LBP 表面呈现。具体来说,我们评估了 LBP 生长如何影响表面呈现的靶向配体的稀释以及随后随着时间的推移特定靶标附着的丧失。接下来,我们描述了可以优化以促进 LBP 靶标附着的关键表面改性参数(例如浓度、停留时间)。然后,我们通过评估 LBP 的生长、活力、储存、对哺乳动物细胞的毒性和体内定植,描述了生物共轭如何影响 LBP 口服给药的适用性。 概括地说,我们描述了影响表面改性 LBP 性能的关键参数,并随后概述了用于表征和评估其口服递送适用性的实验流程。
更新日期:2020-12-30
中文翻译:

用于口服给药的表面改性活生物治疗产品的评价
活生物治疗产品(LBP),包括共生细菌和基因工程细菌,是一类很有前途的新兴疗法,在临床前和临床上对其口服递送至胃肠道(GI)进行了广泛研究。一种新兴的递送策略涉及通过非共价或共价修饰直接功能化 LBP 表面,以控制 LBP 与胃肠道微环境的相互作用,从而提高其活力、附着或治疗效果。然而,与其他治疗方式不同,LBP 是活的有机体,对表面修饰提出了两个独特的挑战:(1)这种方法可以直接干扰关键的 LBP 生物过程(例如,定植、代谢物分泌)和(2)修饰可能因表面修饰而变化。 LBP 表面的动态性质。总的来说,这些因素仍然没有被表征,因为它们与 LBP 的口服给药有关。在此,我们利用之前报道的表面修饰平台来广泛评估和表征 LBP 的表面修饰,该平台能够实现靶向配体的 LBP 表面呈现。具体来说,我们评估了 LBP 生长如何影响表面呈现的靶向配体的稀释以及随后随着时间的推移特定靶标附着的丧失。接下来,我们描述了可以优化以促进 LBP 靶标附着的关键表面改性参数(例如浓度、停留时间)。然后,我们通过评估 LBP 的生长、活力、储存、对哺乳动物细胞的毒性和体内定植,描述了生物共轭如何影响 LBP 口服给药的适用性。 概括地说,我们描述了影响表面改性 LBP 性能的关键参数,并随后概述了用于表征和评估其口服递送适用性的实验流程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号