当前位置:
X-MOL 学术
›
Cell Prolif.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
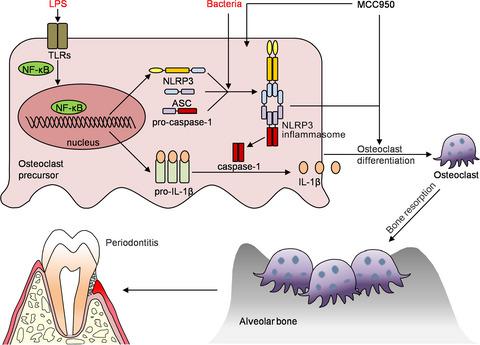
NLRP3 regulates alveolar bone loss in ligature‐induced periodontitis by promoting osteoclastic differentiation
Cell Proliferation ( IF 5.9 ) Pub Date : 2020-12-31 , DOI: 10.1111/cpr.12973 Yuyi Chen 1, 2 , Qiudong Yang 1, 2 , Chunhua Lv 1, 3 , Yue Chen 1, 4 , Wenhua Zhao 1 , Wenlei Li 1 , Hongyu Chen 1 , Hua Wang 1 , Wen Sun 1, 4 , Hua Yuan 1, 2
Cell Proliferation ( IF 5.9 ) Pub Date : 2020-12-31 , DOI: 10.1111/cpr.12973 Yuyi Chen 1, 2 , Qiudong Yang 1, 2 , Chunhua Lv 1, 3 , Yue Chen 1, 4 , Wenhua Zhao 1 , Wenlei Li 1 , Hongyu Chen 1 , Hua Wang 1 , Wen Sun 1, 4 , Hua Yuan 1, 2
Affiliation
|
OBJECTIVES
NLRP3 inflammasome is a critical part of the innate immune system and plays an important role in a variety of inflammatory diseases. However, the effects of NLRP3 inflammasome on periodontitis have not been fully studied. MATERIALS AND METHODS
We used ligature-induced periodontitis models of NLRP3 knockout mice (NLRP3KO ) and their wildtype (WT) littermates to compare their alveolar bone phenotypes. We further used Lysm-Cre/RosanTnG mouse to trace the changes of Lysm-Cre+ osteoclast precursors in ligature-induced periodontitis with or without MCC950 treatment. At last, we explored MCC950 as a potential drug for the treatment of periodontitis in vivo and in vitro. RESULTS
Here, we showed that the number of osteoclast precursors, osteoclast differentiation and alveolar bone loss were reduced in NLRP3KO mice compared with WT littermates, by using ligature-induced periodontitis model. Next, MCC950, a specific inhibitor of the NLRP3 inflammasome, was used to inhibit osteoclast precursors differentiation into osteoclast. Further, we used Lysm-Cre/RosanTnG mice to demonstrate that MCC950 decreases the number of Lysm-Cre+ osteoclast precursors in ligature-induced periodontitis. At last, treatment with MCC950 significantly suppressed alveolar bone loss with reduced IL-1β activation and osteoclast differentiation in ligature-induced periodontitis. CONCLUSION
Our findings reveal that NLRP3 regulates alveolar bone loss in ligature-induced periodontitis by promoting osteoclastic differentiation.
中文翻译:

NLRP3通过促进破骨细胞分化调节结扎诱导的牙周炎中的牙槽骨丢失
目的 NLRP3 炎症小体是先天免疫系统的重要组成部分,在多种炎症性疾病中发挥重要作用。然而,NLRP3炎症小体对牙周炎的影响尚未得到充分研究。材料和方法 我们使用 NLRP3 基因敲除小鼠 (NLRP3KO) 及其野生型 (WT) 同窝仔鼠的结扎诱导牙周炎模型来比较它们的牙槽骨表型。我们进一步使用 Lysm-Cre/RosanTnG 小鼠追踪 Lysm-Cre+ 破骨细胞前体在结扎诱导的牙周炎中有或没有 MCC950 治疗的变化。最后,我们探索了 MCC950 作为体内和体外治疗牙周炎的潜在药物。结果 在这里,我们发现与 WT 同窝小鼠相比,NLRP3KO 小鼠的破骨细胞前体数量、破骨细胞分化和牙槽骨丢失减少,通过使用结扎诱导的牙周炎模型。接下来,使用 NLRP3 炎症小体的特异性抑制剂 MCC950 来抑制破骨细胞前体分化为破骨细胞。此外,我们使用 Lysm-Cre/RosanTnG 小鼠来证明 MCC950 减少了结扎诱导的牙周炎中 Lysm-Cre+ 破骨细胞前体的数量。最后,在结扎诱导的牙周炎中,用 MCC950 治疗显着抑制了牙槽骨丢失,减少了 IL-1β 活化和破骨细胞分化。结论我们的研究结果表明,NLRP3 通过促进破骨细胞分化来调节结扎诱导的牙周炎中的牙槽骨丢失。我们使用 Lysm-Cre/RosanTnG 小鼠来证明 MCC950 减少了结扎诱导的牙周炎中 Lysm-Cre+ 破骨细胞前体的数量。最后,在结扎诱导的牙周炎中,用 MCC950 治疗显着抑制了牙槽骨丢失,减少了 IL-1β 活化和破骨细胞分化。结论我们的研究结果表明,NLRP3 通过促进破骨细胞分化来调节结扎诱导的牙周炎中的牙槽骨丢失。我们使用 Lysm-Cre/RosanTnG 小鼠来证明 MCC950 减少了结扎诱导的牙周炎中 Lysm-Cre+ 破骨细胞前体的数量。最后,在结扎诱导的牙周炎中,用 MCC950 治疗显着抑制了牙槽骨丢失,减少了 IL-1β 活化和破骨细胞分化。结论我们的研究结果表明,NLRP3 通过促进破骨细胞分化来调节结扎诱导的牙周炎中的牙槽骨丢失。
更新日期:2020-12-31
中文翻译:

NLRP3通过促进破骨细胞分化调节结扎诱导的牙周炎中的牙槽骨丢失
目的 NLRP3 炎症小体是先天免疫系统的重要组成部分,在多种炎症性疾病中发挥重要作用。然而,NLRP3炎症小体对牙周炎的影响尚未得到充分研究。材料和方法 我们使用 NLRP3 基因敲除小鼠 (NLRP3KO) 及其野生型 (WT) 同窝仔鼠的结扎诱导牙周炎模型来比较它们的牙槽骨表型。我们进一步使用 Lysm-Cre/RosanTnG 小鼠追踪 Lysm-Cre+ 破骨细胞前体在结扎诱导的牙周炎中有或没有 MCC950 治疗的变化。最后,我们探索了 MCC950 作为体内和体外治疗牙周炎的潜在药物。结果 在这里,我们发现与 WT 同窝小鼠相比,NLRP3KO 小鼠的破骨细胞前体数量、破骨细胞分化和牙槽骨丢失减少,通过使用结扎诱导的牙周炎模型。接下来,使用 NLRP3 炎症小体的特异性抑制剂 MCC950 来抑制破骨细胞前体分化为破骨细胞。此外,我们使用 Lysm-Cre/RosanTnG 小鼠来证明 MCC950 减少了结扎诱导的牙周炎中 Lysm-Cre+ 破骨细胞前体的数量。最后,在结扎诱导的牙周炎中,用 MCC950 治疗显着抑制了牙槽骨丢失,减少了 IL-1β 活化和破骨细胞分化。结论我们的研究结果表明,NLRP3 通过促进破骨细胞分化来调节结扎诱导的牙周炎中的牙槽骨丢失。我们使用 Lysm-Cre/RosanTnG 小鼠来证明 MCC950 减少了结扎诱导的牙周炎中 Lysm-Cre+ 破骨细胞前体的数量。最后,在结扎诱导的牙周炎中,用 MCC950 治疗显着抑制了牙槽骨丢失,减少了 IL-1β 活化和破骨细胞分化。结论我们的研究结果表明,NLRP3 通过促进破骨细胞分化来调节结扎诱导的牙周炎中的牙槽骨丢失。我们使用 Lysm-Cre/RosanTnG 小鼠来证明 MCC950 减少了结扎诱导的牙周炎中 Lysm-Cre+ 破骨细胞前体的数量。最后,在结扎诱导的牙周炎中,用 MCC950 治疗显着抑制了牙槽骨丢失,减少了 IL-1β 活化和破骨细胞分化。结论我们的研究结果表明,NLRP3 通过促进破骨细胞分化来调节结扎诱导的牙周炎中的牙槽骨丢失。











































 京公网安备 11010802027423号
京公网安备 11010802027423号