当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, spectral, crystal structure, drug‐likeness, in silico, and in vitro biological screening of halogen [Cl, Br] substituted N‐phenylbenzo[g]indazole derivatives as antimicrobial agents
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-12-31 , DOI: 10.1002/jhet.4219 S. Murugavel 1 , J. Mohan Raj 1 , C. Ravikumar 2 , R. Ranganathan 3 , G. Jaabil 4 , A. Ponnuswamy 4
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-12-31 , DOI: 10.1002/jhet.4219 S. Murugavel 1 , J. Mohan Raj 1 , C. Ravikumar 2 , R. Ranganathan 3 , G. Jaabil 4 , A. Ponnuswamy 4
Affiliation
|
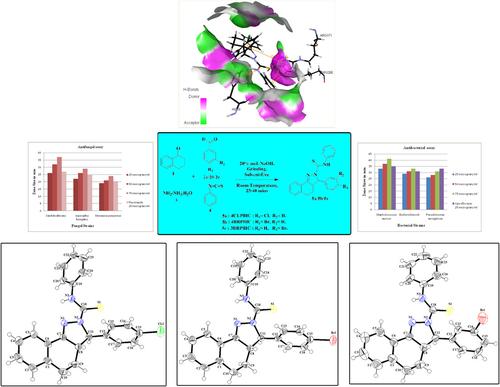
The N‐phenylbenzo[g]indazole derivatives, 3‐(4‐chlorophenyl)‐3,3a,4,5‐tetrahydro‐N‐phenylbenzo[g]indazole‐2‐carbothioamide (4CLPBIC), 3‐(4‐bromophenyl)‐3,3a,4,5‐tetrahydro‐N‐phenylbenzo[g]indazole‐2‐carbothioamide (4BRPBIC), and 3‐(3‐bromophenyl)‐3,3a,4,5‐tetrahydro‐N‐phenylbenzo[g]indazole‐2‐carbothioamide (3BRPBIC), were synthesized by the one‐pot green amalgamation of solvent‐free granulating methodology procedure at room temperature. The synthesized crystals were characterized by single‐crystal X‐ray diffraction (SC‐XRD), FT‐IR, FT‐Raman, NMR, and UV–Vis techniques. The molecular geometries from XRD experimental values of synthesized compounds 4CLPBIC, 4BRPBIC, and 3BRPBIC in the ground state are compared theoretically by applying the density functional theory (DFT), a method with the B3LYP/6‐311G(d,p) basis set using Gaussian 09 software. The vibrational assignments of the synthesized compounds were studied based on potential energy distribution (PED) by the VEDA4 program. The scaled DFT/B3LYP/6‐311G(d,p) results show the best agreement with the experimental values. Computational 1H and 13C NMR were acquired by utilizing gauge‐independent atomic orbital (GIAO) procedure, and chemical shift results are in good agreement with the experimental values. A web‐based theoretical investigation was performed to understand the drug‐likeness and ADMET properties of the compounds. Molecular docking studies were carried out against bacterial cholesterol inhibitor block and inhibitor of lanosterol‐14α‐demethylase CYP51 used in the treatment of topical and systemic mycoses in fungal to understand the inhibitory activity of synthesized compounds. The synthesized molecules were also tested for antibacterial and antifungal activities.
中文翻译:

卤素[Cl,Br]取代的N-苯基苯并[g]吲唑衍生物作为抗菌剂的合成,光谱,晶体结构,药物相似性,计算机模拟和体外生物筛选
所述Ñ -phenylbenzo [克]吲唑衍生物,3-(4-氯苯基)-3,3a,4,5-四氢Ñ -phenylbenzo [克]吲唑-2-硫代甲酰胺(4CLPBIC),3-(4-溴苯基) -3,3a,4,5-四氢-N-苯基苯并[ g ]吲唑-2-碳硫代酰胺(4BRPBIC)和3-(3-溴苯基)-3,3a,4,5-四氢-N-苯基苯并[ g在室温下,通过无溶剂造粒方法的一锅绿色合并,合成]吲唑-2-碳硫酰胺(3BRPBIC)。合成的晶体通过单晶X射线衍射(SC-XRD),FT-IR,FT-Raman,NMR和UV-Vis技术进行表征。应用密度泛函理论(DFT)从理论上比较了合成化合物4CLPBIC,4BRPBIC和3BRPBIC在基态下的XRD实验值的分子几何结构,该方法以B3LYP / 6-311G(d,p)为基础高斯09软件。通过VEDA4程序,基于势能分布(PED)研究了合成化合物的振动分配。缩放后的DFT / B3LYP / 6-311G(d,p)结果显示出与实验值的最佳一致性。计算1H和13 C NMR是通过使用不依赖于轨距的原子轨道(GIAO)程序获得的,化学位移结果与实验值非常吻合。进行了基于网络的理论研究,以了解化合物的相似性和ADMET性质。针对细菌胆固醇抑制剂阻滞剂和羊毛甾醇-14α-脱甲基酶CYP51抑制剂(用于治疗真菌的局部和全身性真菌病)进行了分子对接研究,以了解合成化合物的抑制活性。还测试了合成的分子的抗菌和抗真菌活性。
更新日期:2021-03-02
中文翻译:

卤素[Cl,Br]取代的N-苯基苯并[g]吲唑衍生物作为抗菌剂的合成,光谱,晶体结构,药物相似性,计算机模拟和体外生物筛选
所述Ñ -phenylbenzo [克]吲唑衍生物,3-(4-氯苯基)-3,3a,4,5-四氢Ñ -phenylbenzo [克]吲唑-2-硫代甲酰胺(4CLPBIC),3-(4-溴苯基) -3,3a,4,5-四氢-N-苯基苯并[ g ]吲唑-2-碳硫代酰胺(4BRPBIC)和3-(3-溴苯基)-3,3a,4,5-四氢-N-苯基苯并[ g在室温下,通过无溶剂造粒方法的一锅绿色合并,合成]吲唑-2-碳硫酰胺(3BRPBIC)。合成的晶体通过单晶X射线衍射(SC-XRD),FT-IR,FT-Raman,NMR和UV-Vis技术进行表征。应用密度泛函理论(DFT)从理论上比较了合成化合物4CLPBIC,4BRPBIC和3BRPBIC在基态下的XRD实验值的分子几何结构,该方法以B3LYP / 6-311G(d,p)为基础高斯09软件。通过VEDA4程序,基于势能分布(PED)研究了合成化合物的振动分配。缩放后的DFT / B3LYP / 6-311G(d,p)结果显示出与实验值的最佳一致性。计算1H和13 C NMR是通过使用不依赖于轨距的原子轨道(GIAO)程序获得的,化学位移结果与实验值非常吻合。进行了基于网络的理论研究,以了解化合物的相似性和ADMET性质。针对细菌胆固醇抑制剂阻滞剂和羊毛甾醇-14α-脱甲基酶CYP51抑制剂(用于治疗真菌的局部和全身性真菌病)进行了分子对接研究,以了解合成化合物的抑制活性。还测试了合成的分子的抗菌和抗真菌活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号