Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-12-31 , DOI: 10.1016/j.jsb.2020.107689 Luca Signor 1 , Theo Paris 2 , Caroline Mas 3 , Adrien Picard 2 , Georges Lutfalla 2 , Elisabetta Boeri Erba 1 , Laure Yatime 4
|
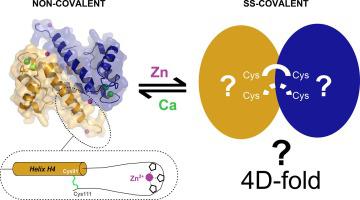
S100A9, with its congener S100A8, belongs to the S100 family of calcium-binding proteins found exclusively in vertebrates. These two proteins are major constituents of neutrophils. In response to a pathological condition, they can be released extracellularly and become alarmins that induce both pro- and anti-inflammatory signals, through specific cell surface receptors. They also act as antimicrobial agents, mainly as a S100A8/A9 heterocomplex, through metal sequestration. The mechanisms whereby divalent cations modulate the extracellular functions of S100A8 and S100A9 are still unclear. Importantly, it has been proposed that these ions may affect both the ternary and quaternary structure of these proteins, thereby influencing their physiological properties. In the present study, we report the crystal structures of WT and C80A murine S100A9 (mS100A9), determined at 1.45 and 2.35 Å resolution, respectively, in the presence of calcium and zinc. These structures reveal a canonical homodimeric form for the protein. They also unravel an intramolecular disulfide bridge that stabilizes the C-terminal tail in a rigid conformation, thus shaping a second Zn-binding site per S100A9 protomer. In solution, mS100A9 apparently binds only two zinc ions per homodimer, with an affinity in the micromolar range, and aggregates in the presence of excess zinc. Using mass spectrometry, we demonstrate that mS100A9 can form both non-covalent and covalent homodimers with distinct disulfide bond patterns. Interestingly, calcium and zinc seem to affect differentially the relative proportion of these forms. We discuss how the metal-dependent interconversion between mS100A9 homodimers may explain the versatility of physiological functions attributed to the protein.
中文翻译:

二价阳离子通过调节其二硫键模式影响鼠 S100A9 蛋白的二聚化模式
S100A9 及其同源物 S100A8 属于仅在脊椎动物中发现的钙结合蛋白 S100 家族。这两种蛋白质是中性粒细胞的主要成分。作为对病理状况的响应,它们可以被释放到细胞外并成为通过特定细胞表面受体诱导促炎和抗炎信号的警报素。它们还通过金属螯合作为抗菌剂,主要作为 S100A8/A9 异质复合物。二价阳离子调节 S100A8 和 S100A9 细胞外功能的机制尚不清楚。重要的是,有人提出这些离子可能会影响这些蛋白质的三元和四元结构,从而影响它们的生理特性。在目前的研究中,我们报告了 WT 和 C80A 鼠 S100A9 (mS100A9) 的晶体结构,在钙和锌存在下,分别以 1.45 和 2.35 Å 分辨率测定。这些结构揭示了该蛋白质的典型同源二聚体形式。他们还解开了一个分子内二硫键,将 C 端尾部稳定在刚性构象中,从而为每个 S100A9 原体形成第二个 Zn 结合位点。在溶液中,mS100A9 显然每个同二聚体仅结合两个锌离子,亲和力在微摩尔范围内,并在过量锌存在下聚集。使用质谱法,我们证明 mS100A9 可以形成具有不同二硫键模式的非共价和共价同二聚体。有趣的是,钙和锌似乎对这些形式的相对比例有不同的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号