Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2020-12-31 , DOI: 10.1016/j.jmgm.2020.107831 Szymon Malinowski 1 , Arghya Pratim Ghosh 2 , Sarah Edwards 3 , Justyna Jaroszynska-Wolinska 4 , Pawel M Kozlowski 2
|
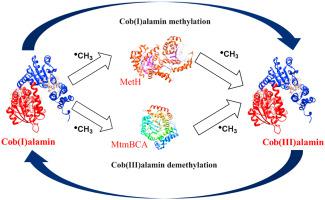
Methyl transfer reactions, mediated by methyltransferases (MeTrs), such as methionine synthase (MetH) or monomethylamine: CoM (MtmBC), constitute one of the most important classes of vitamin B12-dependent reactions. The challenge in exploring the catalytic function of MeTrs is related to their modular structure. From the crystallographic point of view, the structure of each subunit has been determined, but there is a lack of understanding of how each subunit interacts with each other. So far, theoretical studies of methyl group transfer were carried out for the structural models of the active site of each subunit. However, those studies do not include the effect of the enzymatic environment, which is crucial for a comprehensive understanding of enzyme-mediated methyl transfer reactions. Herein, to explore how two subunits interact with each other and how the methyl transfer reaction is catalyzed by MeTrs, molecular docking of the functional units of MetH and MtmBC was carried out. Along with the interactions of the functional units, the reaction coordinates, including the Co–C bond distance for methylation of cob(I)alamin (CoICbl) and the C–S bond distance in demethylation reaction of cob(III)alamin (CoIIICbl), were considered. The functional groups should be arranged so that there is an appropriate distance to transfer a methyl group and present results indicate that steric interactions can limit the number of potential arrangements. This calls into question the possibility of SN2-type mechanism previously proposed for MeTrs. Further, it leads to the conclusion that the methyl transfer reaction involves some spatial changes of modules suggesting an alternate radical-based pathway for MeTrs-mediated methyl transfer reactions. The calculations also showed that changes in torsion angles induce a change in reaction coordinates, namely Co–C and C–S bond distances, for the methylation and demethylation reactions catalyzed both by MetH and MtmBC.
中文翻译:

钴胺素依赖性酶催化的甲基转移反应:分子对接的见解
由甲基转移酶(MeTrs)介导的甲基转移反应,例如蛋氨酸合酶(MetH)或单甲胺:CoM(MtmBC),是维生素B 12最重要的类别之一依赖性反应。探索MeTrs催化功能的挑战与其模块化结构有关。从结晶学的观点来看,已经确定了每个亚基的结构,但是缺乏对每个亚基如何相互作用的理解。到目前为止,对每个亚基活性位点的结构模型进行了甲基转移的理论研究。但是,这些研究并未包括酶促环境的影响,这对于全面理解酶介导的甲基转移反应至关重要。在此,为了探索两个亚基如何相互作用以及如何通过MeTrs催化甲基转移反应,对MetH和MtmBC的功能单元进行了分子对接。随着功能单元的相互作用,考虑了Cob(III)丙氨酸(Co III Cbl)脱甲基反应中的I Cbl)和C–S键距离。官能团的排列方式应使转移甲基的距离合适,目前的结果表明,空间相互作用可限制潜在排列方式的数量。这使人们怀疑S N的可能性以前为MeTrs提出的2型机制。此外,它得出的结论是,甲基转移反应涉及模块的一些空间变化,提示了MeTrs介导的甲基转移反应的基于自由基的替代途径。计算还表明,对于由MetH和MtmBC催化的甲基化和脱甲基反应,扭转角的变化会引起反应坐标的变化,即Co-C和C-S键距。











































 京公网安备 11010802027423号
京公网安备 11010802027423号