Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-12-31 , DOI: 10.1016/j.bmc.2020.115970 Yafei Fan 1 , Hangfei Chen 2 , Ning Mu 1 , Wengui Wang 1 , Kongkai Zhu 3 , Zhi Ruan 2 , Shoufeng Wang 1
|
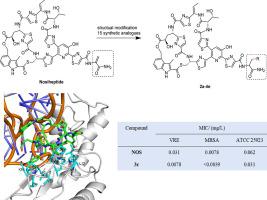
The frequent and inappropriate use of antibiotics aggravate the variation and evolution of multidrug-resistant bacteria, posing a serious threat to public health. Nosiheptide (NOS) has excellent lethality against a variety of Gram-positive bacteria, however the physical and chemical drawbacks hamper its routine application in clinical practice. In this study, by using NOS as the starting material, a total of 15 NOS analogues (2a-4e) were semi-synthesized via its dehydroalanine residue reacting with monosubstituted anilines. In vitro antimicrobial susceptibilities of NOS and its analogues against two methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE) clinical isolates were determined by broth microdilution assay to determine the minimum inhibitory concentration (MIC). Antimicrobial susceptibility testing data shown that most of the NOS analogues had a better antibacterial effect than the parent compound, with compound 3c exhibiting the highest antibacterial activity against VRE (MIC = 0.0078 mg/L) and MRSA (MIC < 0.0039 mg/L). Molecular docking of synthetic compounds was also performed to verify the binding interactions of NOS analogues with the target. Our data indicated that compound 3c possesses stronger and more complex intermolecular force than other analogues, which is consistent with the results of the biological activity evaluation. Overall, this study identified a number of potential antibacterial NOS analogues that could act as potent therapeutic agents for multidrug-resistant bacterial infections.
中文翻译:

那西肽类似物通过脱氢丙氨酸区域修饰作为潜在的抗菌剂:半合成、抗菌活性和分子对接研究
抗生素的频繁使用和不当使用加剧了多重耐药菌的变异和进化,对公众健康构成严重威胁。那西肽(NOS)对多种革兰氏阳性菌具有优异的杀伤力,但其物理和化学缺陷阻碍了其在临床实践中的常规应用。在本研究中,以 NOS 为起始原料,通过其脱氢丙氨酸残基与单取代苯胺反应,共半合成了 15 个 NOS 类似物(2a-4e)。NOS及其类似物对两种耐甲氧西林金黄色葡萄球菌(MRSA)和耐万古霉素屎肠球菌的体外抗菌敏感性(VRE) 临床分离株通过肉汤微量稀释法测定,以确定最低抑菌浓度 (MIC)。抗菌药敏试验数据表明,大多数NOS类似物的抗菌效果优于母体化合物,其中化合物3c对VRE(MIC = 0.0078 mg/L)和MRSA(MIC < 0.0039 mg/L)的抗菌活性最高。还进行了合成化合物的分子对接,以验证 NOS 类似物与靶标的结合相互作用。我们的数据表明化合物3c具有比其他类似物更强、更复杂的分子间作用力,与生物活性评价结果一致。总的来说,这项研究确定了许多潜在的抗菌 NOS 类似物,它们可以作为多重耐药细菌感染的有效治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号