当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel phenolic compounds by DFT: Electronic effects on antioxidant activity of 4-vinylphenol derivatives
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-12-29 , DOI: 10.1002/jccs.202000501 Nazanin Mohebi 1 , Mohamad Z. Kassaee 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-12-29 , DOI: 10.1002/jccs.202000501 Nazanin Mohebi 1 , Mohamad Z. Kassaee 1
Affiliation
|
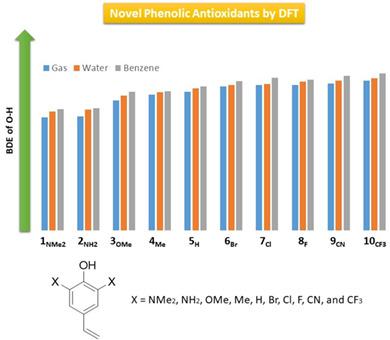
Considering the biological importance of phenolic compounds and their antioxidant activities, we have reached for 10 novel 2,6-diX-4-vinylphenol derivatives (X = NMe2, NH2, OMe, Me, H, Br, Cl, F, CN, and CF3, 1NMe2-10CF3), at the B3LYP/6-311++G** level of theory. To evaluate their antioxidant efficiency, the OH bond dissociation energy (BDE) and vertical ionization potential (IPV) are investigated for all structures in gas, water, and benzene phases, using conductor-like polarized continuum model (CPCM) via B3LYP, LC-ωPBE, M05-2X, and M06-2X functionals. The results indicate that in going from electron-withdrawing groups (EWGs) to electron-donating groups (EDGs), the BDE and IPV values decrease which may suggest the increasing efficiency of antioxidants via hydrogen atom transfer (HAT) and single electron transfer (SET) mechanisms, respectively. The calculated rate constants (krxn) for reactions between 1NMe2-10CF3 with ·OOH and·OH radicals indicate that 1NMe2 shows the highest one. The nucleus-independent chemical shift (NICS) index, energies of highest occupied and lowest unoccupied molecular orbitals (EHOMO and ELUMO, respectively), and natural bond orbital (NBO) analysis provide relevant results to understand the nature of antioxidant activity and stability of their corresponding radicals. The lowest BDE and IPV values are observed in gas and water phases, respectively. Structure 1NMe2 turns out as the most efficient antioxidant for showing the lowest values of BDE and IPV and highest values of NICS, EHOMO, second-order perturbation energy (E2) and natural charge. Spin densities and electrostatic potential (ESP) maps appear consistent with the obtained results. The overall order of antioxidant efficiency in gas, water, and benzene phases is 1NMe2 > 2NH2 >3OMe > 4Me > 5H > 6Br > 7Cl > 8F > 9CN > 10CF3.
中文翻译:

DFT的新型酚类化合物:对4-乙烯基苯酚衍生物抗氧化活性的电子影响
考虑到酚类化合物的生物学重要性及其抗氧化活性,我们已经获得了10种新颖的2,6-diX-4-乙烯基苯酚衍生物(X = NMe 2,NH 2,OMe,Me,H,Br,Cl,F,CN ,和CF 3,1 NME2 - 10 CF3),在B3LYP / 6-311 ++ G **理论水平。为了评估其抗氧化效率,使用了类似导体的极化连续谱模型(CPCM),通过B3LYP研究了气相,水相和苯相中所有结构的OH键解离能(BDE)和垂直电离能(IP V)。 ,LC- ωPBE,M05-2X和M06-2X功能。结果表明,从吸电子基团(EWG)到给电子基团(EDG),BDE和IP V值降低,这可能表明抗氧化剂通过氢原子转移(HAT)和单电子转移的效率有所提高( SET)机制。所计算的速率常数(ķ RXN),用于之间的反应1 NME2 - 10 CF3与·OOH和·OH自由基表明1 NME2示出最高的国家之一。独立于核的化学位移(NICS)指数,最高占据和最低未占据分子轨道的能量(E HOMO和ELUMO和自然键轨道(NBO)分析提供了相关的结果,以了解抗氧化剂活性的性质及其相应自由基的稳定性。在气相和水相中分别观察到最低的BDE和IP V值。结构1 NMe2证明是显示BDE和IP V最低值和NICS,E HOMO,二阶扰动能(E 2)和自然电荷的最高值的最有效的抗氧化剂。自旋密度和静电势(ESP)图看起来与获得的结果一致。气相,水相和苯相中抗氧化剂效率的总体顺序为1NMe 2 > 2 NH 2 > 3 OMe > 4 Me > 5 H > 6 Br > 7 Cl > 8 F > 9 CN > 10 CF 3。
更新日期:2020-12-29
中文翻译:

DFT的新型酚类化合物:对4-乙烯基苯酚衍生物抗氧化活性的电子影响
考虑到酚类化合物的生物学重要性及其抗氧化活性,我们已经获得了10种新颖的2,6-diX-4-乙烯基苯酚衍生物(X = NMe 2,NH 2,OMe,Me,H,Br,Cl,F,CN ,和CF 3,1 NME2 - 10 CF3),在B3LYP / 6-311 ++ G **理论水平。为了评估其抗氧化效率,使用了类似导体的极化连续谱模型(CPCM),通过B3LYP研究了气相,水相和苯相中所有结构的OH键解离能(BDE)和垂直电离能(IP V)。 ,LC- ωPBE,M05-2X和M06-2X功能。结果表明,从吸电子基团(EWG)到给电子基团(EDG),BDE和IP V值降低,这可能表明抗氧化剂通过氢原子转移(HAT)和单电子转移的效率有所提高( SET)机制。所计算的速率常数(ķ RXN),用于之间的反应1 NME2 - 10 CF3与·OOH和·OH自由基表明1 NME2示出最高的国家之一。独立于核的化学位移(NICS)指数,最高占据和最低未占据分子轨道的能量(E HOMO和ELUMO和自然键轨道(NBO)分析提供了相关的结果,以了解抗氧化剂活性的性质及其相应自由基的稳定性。在气相和水相中分别观察到最低的BDE和IP V值。结构1 NMe2证明是显示BDE和IP V最低值和NICS,E HOMO,二阶扰动能(E 2)和自然电荷的最高值的最有效的抗氧化剂。自旋密度和静电势(ESP)图看起来与获得的结果一致。气相,水相和苯相中抗氧化剂效率的总体顺序为1NMe 2 > 2 NH 2 > 3 OMe > 4 Me > 5 H > 6 Br > 7 Cl > 8 F > 9 CN > 10 CF 3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号