当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption Mechanism of Bentonite with Dispersed Chitosan for Cadmium Ions
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2020-12-29 , DOI: 10.1002/ceat.202000505 Chengdu Huang 1 , Yongchun Huang 1 , Tenghui Xie 1 , Wanguo Yu 1 , Shuo Ai 1
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2020-12-29 , DOI: 10.1002/ceat.202000505 Chengdu Huang 1 , Yongchun Huang 1 , Tenghui Xie 1 , Wanguo Yu 1 , Shuo Ai 1
Affiliation
|
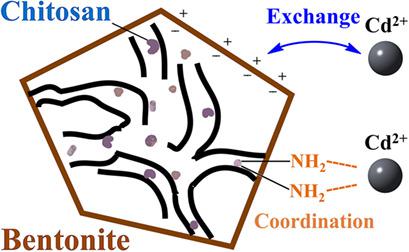
Chitosan was dispersed on bentonite for the preparation of a Cd2+ adsorbent. The adsorption process followed a Freundlich isotherm, and the experimental adsorption capacity was determined. For understanding the adsorption mechanism, the pristine and Cd‐doped adsorbents were analyzed by Fourier transform infrared and X‐ray photoelectron spectroscopy. A red shift of the band corresponding to amino groups was observed, while the Cd 3d binding energy declined. Furthermore, density functional theory calculation results indicated that Cd2+ bonded with amino and hydroxyl groups in chitosan. The zeta potential of the adsorbent was lower than that of the support bentonite, improving the electrostatic affinity for Cd2+. The synergy between electrostatic and coordination interactions contributed to the adsorption capacity.
中文翻译:

膨润土与分散的壳聚糖对镉离子的吸附机理
将壳聚糖分散在膨润土上以制备Cd 2+吸附剂。吸附过程遵循Freundlich等温线,并确定了实验吸附容量。为了了解吸附机理,通过傅里叶变换红外和X射线光电子能谱分析了原始和Cd掺杂的吸附剂。观察到对应于氨基的条带的红移,而Cd 3d的结合能下降。此外,密度泛函理论计算结果表明,Cd 2+与壳聚糖中的氨基和羟基键合。吸附剂的ζ电势低于载体膨润土的ζ电势,提高了对Cd 2+的静电亲和力。静电和配位相互作用之间的协同作用有助于吸附能力。
更新日期:2021-02-19
中文翻译:

膨润土与分散的壳聚糖对镉离子的吸附机理
将壳聚糖分散在膨润土上以制备Cd 2+吸附剂。吸附过程遵循Freundlich等温线,并确定了实验吸附容量。为了了解吸附机理,通过傅里叶变换红外和X射线光电子能谱分析了原始和Cd掺杂的吸附剂。观察到对应于氨基的条带的红移,而Cd 3d的结合能下降。此外,密度泛函理论计算结果表明,Cd 2+与壳聚糖中的氨基和羟基键合。吸附剂的ζ电势低于载体膨润土的ζ电势,提高了对Cd 2+的静电亲和力。静电和配位相互作用之间的协同作用有助于吸附能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号