当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and Computational Study on the Intramolecular Hydrogen Atom Transfer Reactions of Maleimide-Based Enediynes After Cycloaromatization
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-29 , DOI: 10.1021/acs.joc.0c02401 Mengsi Zhang 1 , Haotian Lu 1 , Baojun Li 1 , Hailong Ma 1 , Wenbo Wang 1 , Xiaoyu Cheng 1 , Yun Ding 1 , Aiguo Hu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-29 , DOI: 10.1021/acs.joc.0c02401 Mengsi Zhang 1 , Haotian Lu 1 , Baojun Li 1 , Hailong Ma 1 , Wenbo Wang 1 , Xiaoyu Cheng 1 , Yun Ding 1 , Aiguo Hu 1
Affiliation
|
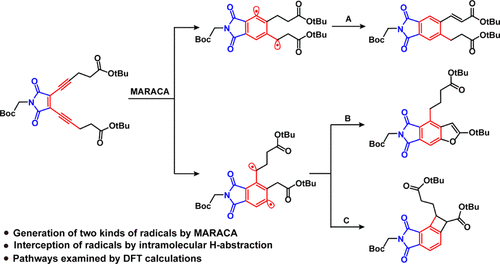
The follow-up reaction pathways of the diradical species formed from cycloaromatization of enediynes or enyne–allenes determine their ability of H-abstraction from DNA, significantly affecting their biological activity performance. To gain a deeper understanding of subsequent reaction pathways of the diradical intermediates formed from acyclic enediynes based on maleimide-assisted rearrangement and cycloaromatization (MARACA), a maleimide-based enediyne featuring methylene groups adjacent to the propargyl sites of the terminal alkynes was synthesized through the Sonogashira coupling reaction. Three thermal cyclization products after intramolecular hydrogen atom transfer (HAT) were obtained from the thermolysis experiment and their structures were confirmed by 1D and 2D nuclear magnetic resonance spectroscopic analysis. Density functional theory was employed to analyze the important elementary steps including rearrangement, cycloaromatization, and intramolecular HAT processes toward the formation of the cyclized products, where the low-energy barriers of HAT pathways relative to the formation of diradicals from cycloaromatization were successfully identified. Overall, the HAT processes to consume diradicals intramolecularly have become competitive with that of intermolecular H-abstraction, implying that the DNA-cleavage ability of enediynes can be further boosted once the HAT processes are halted. This study offers a promising direction for designing novel and potent acyclic enediynes for antitumor applications.
中文翻译:

环芳香化后马来酰亚胺基对映体分子内氢原子转移反应的实验和计算研究
烯二炔或烯炔-丙二烯环芳烃化形成的双自由基物种的后续反应途径决定了它们从DNA中提取H的能力,从而极大地影响了其生物学活性。为了更深入地了解基于马来酰亚胺辅助的重排和环芳构化(MARACA)的由无环烯二炔形成的双自由基中间体的后续反应途径,合成了一种基于马来酰亚胺的烯二炔,其特征在于与末端炔基的炔丙基位相邻的亚甲基通过Sonogashira偶联反应。通过热解实验获得了分子内氢原子转移(HAT)后的三种热环化产物,并通过一维和二维核磁共振光谱分析确定了它们的结构。密度泛函理论用于分析重要的基本步骤,包括重排,环芳族化和分子内HAT过程,以形成环化产物,其中成功鉴定了HAT途径相对于环芳族化形成双自由基的低能垒。总的来说,HAT分子内消耗双自由基的过程已经与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。分子内的HAT过程朝着环化产物的形成方向发展,其中成功鉴定了HAT通路相对于由环芳构化形成双自由基形成的低能垒。总的来说,HAT分子内消耗双自由基的过程已经与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。分子内的HAT过程朝着环化产物的形成方向发展,其中成功鉴定了HAT通路相对于由环芳构化形成双自由基形成的低能垒。总的来说,HAT分子内消耗双自由基的过程已经与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。HAT分子内消耗双自由基的过程已与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。HAT分子内消耗双自由基的过程已与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。
更新日期:2021-01-16
中文翻译:

环芳香化后马来酰亚胺基对映体分子内氢原子转移反应的实验和计算研究
烯二炔或烯炔-丙二烯环芳烃化形成的双自由基物种的后续反应途径决定了它们从DNA中提取H的能力,从而极大地影响了其生物学活性。为了更深入地了解基于马来酰亚胺辅助的重排和环芳构化(MARACA)的由无环烯二炔形成的双自由基中间体的后续反应途径,合成了一种基于马来酰亚胺的烯二炔,其特征在于与末端炔基的炔丙基位相邻的亚甲基通过Sonogashira偶联反应。通过热解实验获得了分子内氢原子转移(HAT)后的三种热环化产物,并通过一维和二维核磁共振光谱分析确定了它们的结构。密度泛函理论用于分析重要的基本步骤,包括重排,环芳族化和分子内HAT过程,以形成环化产物,其中成功鉴定了HAT途径相对于环芳族化形成双自由基的低能垒。总的来说,HAT分子内消耗双自由基的过程已经与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。分子内的HAT过程朝着环化产物的形成方向发展,其中成功鉴定了HAT通路相对于由环芳构化形成双自由基形成的低能垒。总的来说,HAT分子内消耗双自由基的过程已经与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。分子内的HAT过程朝着环化产物的形成方向发展,其中成功鉴定了HAT通路相对于由环芳构化形成双自由基形成的低能垒。总的来说,HAT分子内消耗双自由基的过程已经与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。HAT分子内消耗双自由基的过程已与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。HAT分子内消耗双自由基的过程已与分子间H吸收竞争,这意味着一旦HAT过程停止,烯二炔的DNA裂解能力将进一步提高。这项研究为设计用于抗肿瘤应用的新型有效的无环烯二炔提供了有希望的方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号