当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combining Rational Design and Continuous Evolution on Minimalist Proteins That Target the E-box DNA Site
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-12-28 , DOI: 10.1021/acschembio.0c00684 Ichiro Inamoto 1 , Inder Sheoran 1 , Serban C Popa 1 , Montdher Hussain 1 , Jumi A Shin 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-12-28 , DOI: 10.1021/acschembio.0c00684 Ichiro Inamoto 1 , Inder Sheoran 1 , Serban C Popa 1 , Montdher Hussain 1 , Jumi A Shin 1
Affiliation
|
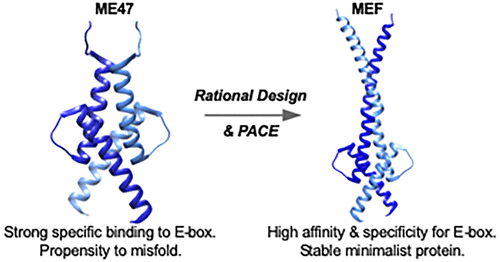
Protein-based therapeutics are part of the next-generation arsenal of drugs being developed against proto-oncoprotein Myc. We designed protein MEF to mimic the basic region/helix-loop-helix/leucine zipper (bHLHZ) domain of Max and Myc, which bind to the E-box motif (enhancer box, CACGTG). To make MEF, we started with our rationally designed ME47, a hybrid of the Max basic region and E47 HLH, that effectively inhibited tumor growth in a mouse model of breast cancer. We used phage-assisted continuous evolution (PACE), which uncovered mutations at Arg12 that contact the DNA phosphodiester backbone. The Arg12 mutations improved ME47’s stability. We replaced Cys29 with Ala to eliminate potential undesired disulfide formation and fused the designed FosW leucine zipper to mutated ME47 to increase the dimerization interface and E-box targeting activity. This “franken-protein” MEF comprises the Max basic region, E47 HLH, and FosW leucine zipper. Compared with ME47, MEF gives 2-fold stronger binding to E-box and 4-fold increased specificity for E-box over nonspecific DNA. The synergistic combination of rational design and PACE allowed us to make MEF and demonstrates the power and utility of our two-pronged approach toward development of promising protein drugs with robust structure and DNA-binding function.
中文翻译:

结合针对E-box DNA位点的极简蛋白质的合理设计和持续进化
基于蛋白质的疗法是针对原癌蛋白Myc开发的新一代药物库的一部分。我们设计了蛋白质MEF,以模仿Max和Myc的基本区域/螺旋-环-螺旋/亮氨酸拉链(bHLHZ)域,该域与E-box主题(增强子盒,CACGTG)结合。制作MEF,我们从合理设计的ME47(Max基本区域和E47 HLH的混合体)开始,它可以有效抑制乳腺癌小鼠模型中的肿瘤生长。我们使用了噬菌体辅助连续进化(PACE)技术,该技术在Arg12处发现了与DNA磷酸二酯主链接触的突变。Arg12突变提高了ME47的稳定性。我们用Ala取代Cys29,以消除潜在的不希望的二硫键形成,并将设计的FosW亮氨酸拉链与突变的ME47融合,以增加二聚化界面和E-box靶向活性。该“弗兰肯蛋白” MEF包含最大碱性区域,E47 HLH和FosW亮氨酸拉链。与ME47,MEF相比与E-box的结合力比非特异性DNA高2倍,对E-box的特异性提高4倍。合理设计和PACE的协同结合使我们能够制造MEF,并证明了我们两管齐下的方法在开发具有鲁棒结构和DNA结合功能的有前途的蛋白质药物方面的强大功能和实用性。
更新日期:2021-01-15
中文翻译:

结合针对E-box DNA位点的极简蛋白质的合理设计和持续进化
基于蛋白质的疗法是针对原癌蛋白Myc开发的新一代药物库的一部分。我们设计了蛋白质MEF,以模仿Max和Myc的基本区域/螺旋-环-螺旋/亮氨酸拉链(bHLHZ)域,该域与E-box主题(增强子盒,CACGTG)结合。制作MEF,我们从合理设计的ME47(Max基本区域和E47 HLH的混合体)开始,它可以有效抑制乳腺癌小鼠模型中的肿瘤生长。我们使用了噬菌体辅助连续进化(PACE)技术,该技术在Arg12处发现了与DNA磷酸二酯主链接触的突变。Arg12突变提高了ME47的稳定性。我们用Ala取代Cys29,以消除潜在的不希望的二硫键形成,并将设计的FosW亮氨酸拉链与突变的ME47融合,以增加二聚化界面和E-box靶向活性。该“弗兰肯蛋白” MEF包含最大碱性区域,E47 HLH和FosW亮氨酸拉链。与ME47,MEF相比与E-box的结合力比非特异性DNA高2倍,对E-box的特异性提高4倍。合理设计和PACE的协同结合使我们能够制造MEF,并证明了我们两管齐下的方法在开发具有鲁棒结构和DNA结合功能的有前途的蛋白质药物方面的强大功能和实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号