Chemical Data Collections Pub Date : 2020-12-29 , DOI: 10.1016/j.cdc.2020.100644 V. Arjunan , L. Devi , S. Mohan
|
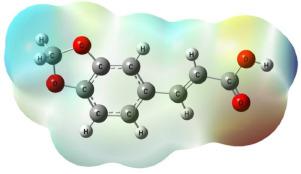
The molecular structural parameters and thermodynamic properties of trans–3,4–(methylenedioxy)cinnamic acid have been determined by B3LYP method by using 6–311++G** and cc–pVTZ basis sets. The vibrational frequencies of the compound have been precisely assigned, analysed and the theoretical results are compared with FT-IR and FT-Raman data. The energies of important MOs of the compound are also evaluated from DFT method. The frontier orbital energy gap (ELUMO–EHOMO) is found to be 4.1032 eV. The hydrogen and carbon chemical shifts are elaborately discussed. The dual descriptors Δfk, Δsk and Δωk reveals that the order of reactivity towards nucleophilic attack is C1 > O13 > O12 > C3 > C8 > C6. The minimum and maximum limits of the electrostatic potential observed in MDCA is –1.448e × 10–2(red) and +1.448e × 10–2 (blue). Thus, the present investigation provides complete structural, vibrational and reactivity characteristics of the compound .
中文翻译:

反式–3,4–(亚甲二氧基)肉桂酸的能谱,结构,光谱和量子化学研究
反式–3,4– (亚甲二氧基)肉桂酸的分子结构参数和热力学性质已通过B3LYP方法使用6–311 ++ G **和cc–pVTZ基组进行了测定。化合物的振动频率已精确分配,分析,并将理论结果与FT-IR和FT-Raman数据进行了比较。化合物的重要MO的能量也可以通过DFT方法进行评估。发现边界轨道能隙(E LUMO –E HOMO)为4.1032 eV。详细讨论了氢和碳的化学位移。双描述的Δf ķ,△S ķ和Δω ķ揭示对亲核攻击的反应性顺序为C1> O13> O12> C3> C8> C6。在MDCA中观察到的静电势的最小和最大限制为–1.448e×10 –2(红色)和+ 1.448e×10 –2(蓝色)。因此,本研究提供了该化合物的完整的结构,振动和反应性特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号