Structure ( IF 4.4 ) Pub Date : 2020-12-29 , DOI: 10.1016/j.str.2020.12.002 Wenguang G Liang 1 , Jordan M Mancl 1 , Minglei Zhao 2 , Wei-Jen Tang 1
|
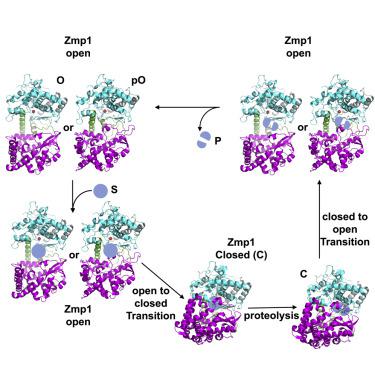
Zinc metalloprotease 1 (Zmp1), a Mycobacterium tuberculosis 75 kDa secreted enzyme, mediates key stages of tuberculosis disease progression. The biological activity of Zmp1 presumably stems from its ability to degrade bacterium- and/or host-derived peptides. The crystal structures of Zmp1 and related M13 metalloproteases, such as neprilysin and endothelin-converting enzyme-1 were determined only in the closed conformation, which cannot capture substrates or release proteolytic products. Thus, the mechanisms of substrate binding and selectivity remain elusive. Here we report two open-state cryo-EM structures of Zmp1, revealed by our SAXS analysis to be the dominant states in solution. Our structural analyses reveal how ligand binding induces a conformational switch in four linker regions to drive the rigid body motion of the D1 and D2 domains, which form the sizable catalytic chamber. Furthermore, they offer insights into the catalytic cycle and mechanism of substrate recognition of M13 metalloproteases for future therapeutic innovations.
中文翻译:

结核分枝杆菌 M13 金属蛋白酶 Zmp1 开放状态的结构分析
锌金属蛋白酶 1 (Zmp1),一种结核分枝杆菌75 kDa 分泌酶,介导结核病进展的关键阶段。Zmp1 的生物活性可能源于其降解细菌和/或宿主衍生肽的能力。Zmp1 和相关 M13 金属蛋白酶的晶体结构,如脑啡肽酶和内皮素转换酶-1 仅在闭合构象中确定,不能捕获底物或释放蛋白水解产物。因此,底物结合和选择性的机制仍然难以捉摸。在这里,我们报告了 Zmp1 的两种开放状态低温电磁结构,通过我们的 SAXS 分析显示为溶液中的主要状态。我们的结构分析揭示了配体结合如何在四个接头区域中诱导构象转换,以驱动 D1 和 D2 结构域的刚体运动,从而形成相当大的催化室。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号