Inorganic Chemistry Communications ( IF 4.4 ) Pub Date : 2020-12-29 , DOI: 10.1016/j.inoche.2020.108409 K. Dahmani , M. Galai , M. Ouakki , M. Cherkaoui , R. Touir , S. Erkan , S. Kaya , B. El Ibrahimi
|
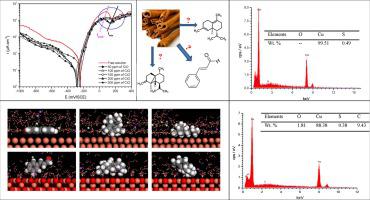
The influence of the extracted cinnamon essential oil (CiO) on the copper corrosion resistance in acidified 3.0 wt% NaCl (pH = 2) medium was investigated by using electrochemical measurements and Scanning Electron Microscopy (SEM) with Energy Dispersive X-Ray Analysis (EDS). So, in order to identify the component of CiO responsible for corrosion inhibition of copper in corrosive solution, the density functional theory (DFT) calculations and molecular dynamics simulation were used. Potentiodynamic polarization showed that the tested natural product acts as cathodic-type inhibitor. Electrochemical impedance spectroscopy indicated that the inhibition efficiency increases with CiO concentrations to get up a maximum value of 89% at 200 ppm. In addition, SEM/EDS analysis in the presence of 200 ppm CiO indicated that copper surface was exempt for all corrosion products, confirming its offered protection. Finally, the major calculated quantum chemical descriptors obtained from DFT calculations indicated that the anticorrosion efficiency responsibility attributes to P8 and P46, with the predominance of P8. In the same, the molecular dynamics simulation indicated that the adsorption energy follows the order: P5 (-61.071 kJ mol−1) > P46 (-58.070 kJ mol−1) > P8 (-42.938 kJ mol−1) on Cu (1 1 1) and P8 (-21.220 kJ mol−1) > P46 (-20.066 kJ mol−1) > P5 (-19.591 kJ mol−1) on CuO2 (1 1 0), suggesting a strong adsorption of P8 on oxide copper (1 1 0) surface and consequently therefore the performance of extracted CiO can be attributed to P8, which is parallel to the copper (1 1 1) surface contrary to other molecules P46 and P5.
中文翻译:

用于鉴定提取的肉桂精油成分的量子化学和分子动力学模拟研究,该成分可在酸化的3.0 wt%NaCl介质中抑制铜腐蚀
通过电化学测量和带能量色散X射线分析(EDS)的扫描电子显微镜(SEM),研究了提取的肉桂精油(CiO)对酸化的3.0 wt%NaCl(pH = 2)介质中铜耐腐蚀性的影响。 )。因此,为了确定腐蚀溶液中负责抑制铜腐蚀的CiO成分,使用了密度泛函理论(DFT)计算和分子动力学模拟。电位动力学极化表明,所测试的天然产物充当阴极型抑制剂。电化学阻抗谱表明,抑制效率随CiO浓度的增加而增加,在200 ppm时达到89%的最大值。此外,在200 ppm CiO存在下的SEM / EDS分析表明,所有腐蚀产物均不包含铜表面,从而证实了其提供的保护作用。最后,从DFT计算获得的主要计算出的量子化学描述符表明,防腐效率的责任归因于P8和P46,其中P8占主导地位。同样,分子动力学模拟表明吸附能遵循以下顺序:P5(-61.071 kJ mol-1)> P46(-58.070 kJ mol -1)> P8(-42.938 kJ mol -1)在Cu(1 1 1)和P8(-21.220 kJ mol -1)> P46(-20.066 kJ mol -1) > CuO 2(1 1 0 )上的P5(-19.591 kJ mol -1),表明P8在氧化物铜(1 1 0)表面上的强烈吸附,因此,提取的CiO的性能可归因于P8,即平行于铜(1 1 1)表面,与其他分子P46和P5相反。










































 京公网安备 11010802027423号
京公网安备 11010802027423号